

Chinese Journal of Organic Chemistry >
Synthesis and Biological Evaluation of Novel Flavonoid-Substituted Tröger's Bases
Received date: 2018-05-03
Revised date: 2018-07-27
Online published: 2018-09-05
Supported by
Project supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions, the Major Project of the Natural Science Research of University in Jiangsu Province (No. 15KJA180002) and the Aid Project for PhD Faculties in Jiangsu Normal University (No. 17XLR023).
A series of flavonoid-substituted Tröger's base analogues were synthesized via multi-step reaction. Their anti-cancer activities on the HepG2 hepatocellular carcinoma cell and antibacterial activities on four bacterial (Pseudomonas aeruginosa PAM1032, wild type Staphylococcus aureus, wild type Escherichia coli and Escherichia.coli-NMD-1) were evaluated. Two compounds were screened out because of their high inhibitory rate on Staphylococcus aureus at 1 μg/mL. The IC50 values of five products on the HepG2 (hepatocellular carcinoma cell) were lower than that of positive control paclitaxel (30.87 μg/mL), displaying their high inhibitory activity. The results indicated their potential applications in new drug development.
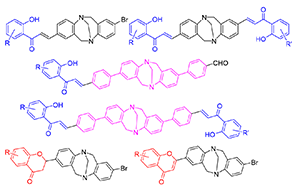
Key words: Tröger's base; flavonoid; bioactivity
Yuan Rui , Wang Yuanjiang , Huang Shuying , Dou Pengfei , Zhang Longyan , Chen Wen , Ren Xuanxuan , Zhou Shengliang , Wan Yu , Wu Hui . Synthesis and Biological Evaluation of Novel Flavonoid-Substituted Tröger's Bases[J]. Chinese Journal of Organic Chemistry, 2018 , 38(12) : 3338 -3344 . DOI: 10.6023/cjoc201805011
[1] Tröger, J. J. Prakt. Chem. 1887, 36, 225.
[2] Prelog, V.; Wieland, P. Helv. Chim. Acta 1944, 27, 1127.
[3] Spielman, M. A. J. Am. Chem. Soc. 1935, 57, 583.
[4] Wilen, S. H.; Qi, J. Z.; Williard, P. G. J. Org. Chem. 1991, 56, 485.
[5] Wilcox, C. S. Tetrahedron Lett. 1985, 26, 5749.
[6] Harmata, M.; Onrayanil, K.; Barnes, C. L. Supramol. Chem. 2006, 18, 581.
[7] Sergeyev, S. Chim. Acta 2009, 92, 415.
[8] Dolensky, B.; Elguero, J.; Král, V.; Pardo, C.; Valík, M. Het-erocycl. Chem. 2007, 93, 1.
[9] Yuan, C. X.; Xin, Q.; Liu, H. J.; Wang, L.; Jiang, M. H.; Tao, X. T. Sci. China Chem. 2011, 54, 587.
[10] Pardo, C.; Sesmilo, E.; Gutiérrez-Puebla, E.; Monge, A.; El-guero, J. J. Org. Chem. 2001, 66, 1607.
[11] Bailly, C.; Laine, W.; Demeunynck, M.; Lhomme, J. Biochem. Bioph. Res. Commun. 2000, 273, 681.
[12] Marrero-Morejon, J.; Pardillo-Fontdevila, E.; Fernan-dez-Benitez, S. Chem. Commun. 1999, 161.
[13] Veale, E. B.; Frimannsson, D. O.; Lawler, M.; Gunnlaugsson, T. Org. Lett. 2009, 11, 4040.
[14] Johnson, R. A.; Gorman, R. R.; Wnuk, R. J.; Crittenden, N. J. J. Med. Chem. 1993, 36, 3202.
[15] Blaser, H. U.; Jalett, H. P.; Lottenbach, W.; Studer, M. J. Am. Chem. Soc. 2000, 122, 12675.
[16] Taylor, L. P.; Grotewold, E. Plant Biol. 2005, 8, 317.
[17] Pier-Giorgio P. J. Nat. Prod. 2000, 63, 1035.
[18] Bravo, L. Nutr. Rev. 1998, 56, 317.
[19] Yang, C. S. Nutrition 1999, 15, 946.
[20] Sun, A. Y.; Simonyi, A.; Sun, G. Y. Free Radical Biol. Med. 2002, 32, 314.
[21] Arts, I. C.; Hollman, P. C. J. Clin. Nutr. 2005, 81, 317S.
[22] Scalbert, A.; Manach, C.; Morand, C.; Remesy, C.; Jimenez, L. Crit. Rev. Food Sci. Nutr. 2005, 45, 287.
[23] Yuan, R.; Li, M. Q.; Xu, J. B.; Huang, S. Y.; Zhou, S. L.; Zhang, P.; Liu, J. -J.; Wu, H. Tetrahedron 2016, 72, 4081.
[24] Chu, Y. H.; Wan, Y.; Liu, Z. T.; Qiu, F.; Han, X. E.; Wu, H. Tetrahedron Lett. 2015, 56, 7046.
[25] Neogi, I.; Jhulki, S.; Ghosh, A.; Chow, T. J.; Moorthy, J. N. Org. Electron. 2014, 15, 3766.
[26] Solano, C.; Svensson, D.; Olomi, Z.; Jensen, J.; Wendt, O. F.; Wärnmark, K. Eur. J. Org. Chem. 2005, 16, 3510.
[27] Jensen, J.; Tejler, J.; Waernmark, K. J. Org. Chem. 2002, 67, 6008.
[28] Safavi, M.; Esmati, N.; Ardestani, S. K.; Emami, S.; Ajdari, S.; Davoodi, J.; Shafiee, A.; Foroumadi, A. Eur. J. Med. Chem. 2012, 58, 573.
[29] Mai, C. W.; Yaeghoobi, M.; Abd-Rahman, N.; Kang, Y. B.; Pichika, M. R. Eur. J. Med. Chem. 2014, 77, 378.
/
| 〈 |
|
〉 |