

Chinese Journal of Organic Chemistry >
Palladium Catalyzed Allylic Amination of Cinnamyl Carbonates with Acyl Hydrazones
Received date: 2018-08-13
Revised date: 2018-09-03
Online published: 2018-09-10
Supported by
Project supported by the National Natural Science Foundation of China (No. 21572126), the Program for Science & Technology Innovation Talents in Universities of Henan Province (No. 14HASTIT016) and the Program of Science and Technology Innovation Talents of Henan Province (No. 2018JQ0011).
Allylic amines moiety exists extensively in natural products, medicines and functional materials. In addition, they are also a kind of versatile building blocks for organic synthesis. Using CH3CN as solvent, the palladium catalyzed allyl amination of cinnamyl carbonate and acylhydrazone compounds was realized under argon. The linear product was formed selectively and the up to 99% yield was obtained. The reaction has features of base free, mild reaction condition, simple operation and broad substrate scope.
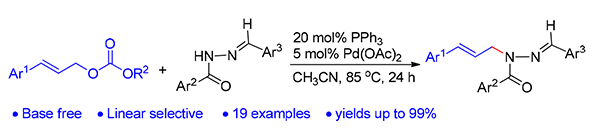
Key words: paladium catalysis; allylic amination; cinnamyl carbonate
Liu Lantao , Chen Yingyinga , Zhang An'an , Liu Xue , Zhang Li , Bai Jingru , Li Heng , Mao Guoliang . Palladium Catalyzed Allylic Amination of Cinnamyl Carbonates with Acyl Hydrazones[J]. Chinese Journal of Organic Chemistry, 2019 , 39(2) : 475 -481 . DOI: 10.6023/cjoc201808013
[1] (a) Brown, E. G. Ring Nitrogen and Key Biomolecules, Springer, Boston, MA, 1998.
(b) Yu, X. Y.; Zhou, F.; Chen, J. R.; Xiao, W. J. Acta Chim. Sinica 2017, 75, 86(in Chinese). (余晓叶, 周帆, 陈加荣, 肖文精, 化学学报, 2017, 75, 86.)
(c) Li, W. F.; Ma, Q.; Zheng, Z. Z.; Zhang, Y. G. Acta Chim. Sinica 2017, 75, 225(in Chinese). (李宛飞, 马倩, 郑召召, 张跃钢, 化学学报, 2017, 75, 225.)
(d) Li, T. T.; Zhao, J. K.; Li, R.; Quan, Z. L.; Xu, J. Acta Chim. Sinica 2017, 75, 485(in Chinese). (李甜甜, 赵继宽, 李尧, 全贞兰, 徐洁, 化学学报, 2017, 75, 485.)
[2] (a) Trost, B. M.; Crawley, M. L. Chem. Rev. 2003, 103, 2921.
(b) Trost, B. M.; Pissot-Soldermann, C.; Chen, I.; Schroede, G. M. J. Am. Chem. Soc. 2004, 126, 4480.
(c) Wang, D.; Yu, X.; Ge, B.; Miao, H.; Ding, Y. Chin. J. Org. Chem. 2015, 35, 676(in Chinese). (王大伟, 余晓丽, 葛冰洋, 苗红艳, 丁玉强, 有机化学, 2015, 35, 676.)
(d) Hu, X.; Yang, B.; Yao, W.; Wang, D. Chin. J. Org. Chem. 2018, 38, 3296(in Chinese). (胡欣宇, 杨伯斌, 姚玮, 王大伟, 有机化学, 2018, 38, 3296.)
[3] (a) Monk, J. P.; Brogden, R. N. Drugs 1991, 42, 659.
(b) Birnbaum, J. E. J. Am. Acad. Dermatol. 1990, 23, 782.
(c) Xu, Z.; Wang, D. S.; Yu, X.; Yang Y.; Wang, D. Adv. Synth. Catal. 2017, 359, 3332.
[4] (a) Stuetz, A.; Petranyi, G. J. Med. Chem. 1984, 27, 1539.
(b) Rudisill, D. E.; Castonguay, L. A.; Me, J. K. Tetrahedron Lett. 1988, 29, 1509.
(c) Balfour, J. A.; Fauids, D. Drugs 1992, 43, 259.
(d) Ge, C.; Sang, X.; Yao, W.; Zhang L.; Wang, D. Green Chem. 2018, 20, 1805.
[5] (a) Andersson, P. G.; Backvall, J. E. In Handbook of Organopalladium Chemistry for Organic Synthesis, Ed.:Negishi, E., Wiley-Interscience, New York, 2002, p. 1859.
(b) Davies, H. M. L.; Long, M. S. Angew. Chem., Int. Ed. 2005, 44, 3518.
(c) Jiang, L.; Buchwald, S. L. In Metal-Catalyzed Cross-Coupling Reactions, 2nd ed., Wiley-VCH, Weinheim, 2004, Vol. 2, p. 699.
(d) Hartwig, J. F. In Handbook of Organopalladium Chemistry for Organic Synthesis, Ed.:Negishi, E., Wiley-Interscience, New York, 2002, p. 1051.
[6] (a) Ranerand, K. D.; Ward, A. D. Aust. J. Chem. 1991, 44, 1749.
(b) Cooper, M. A.; Lucas, M. A.; Taylor, J. M.; Ward, A. D.; Williamson, N. M. Synthesis 2001, 621.
(c) Vicente, R. Org. Biomol. Chem. 2011, 9, 6469.
[7] (a) Schultz, D. M.; Wolfe, J. P. Org. Lett. 2010, 12, 1028.
(b) Caddick, S.; Koe, W. Tetrahedron Lett. 2002, 43, 9347.
(c) Sharma, V.; Kumar, P.; Pathak, D. J. Heterocycl. Chem. 2010, 47, 491.
(d) Lu, T.; Lu, Z.; Ma, Z. X.; Zhang, Y.; Hsung, R. P. Chem. Rev. 2013, 113, 4862.
[8] Cho, C. S.; Kim, J. S.; Oh, B. H. Tetrahedron 2000, 56, 7747.
[9] Cui, X. M. Chlor-Alkali Ind. 2000, 5, 172192(in Chinese). (崔小明, 氯碱工业, 2000, 5, 17219.)
[10] (a) Müller, T. E.; Hultzsch, K. C.; Yus, M.; Foubelo, F.; Tada, M. Chem. Rev. 2008, 108, 3795.
(b) Lu, Z.; Ma, S. M. Angew. Chem., Int. Ed. 2008, 47, 258.
(c) Luo, J.; Jiang, H. F. Chin. J. Org. Chem. 2008, 28, 187(in Chinese). (罗洁, 江焕峰, 有机化学, 2008, 28, 187.)
(d) Li, Y.; Zheng, Y.; Tian, F.; Zhang, Y. J.; Zhang, W. Chin. J. Org. Chem. 2009, 29, 1487(in Chinese). (李亚玺, 郑玉林, 田丰涛, 张勇健, 张万斌, 有机化学, 2009, 29, 1487.)
(e) Lee, O. Y.; Law, K. L.; Yang, D. Org. Lett. 2009, 11, 3302.
(f) Huang, L.; Arndt, M.; Gooßen, K.; Heydt, H.; Gooßen, L. J. Chem. Rev. 2015, 115, 2596.
(g) Butt, N. A.; Zhang, W. Chem. Soc. Rev. 2015, 44, 7929.
[11] Lee, O. Y.; Law, K. L.; Yang, D. Org. Lett. 2009, 11, 3302.
[12] (a) Patel, S. J.; Jamison, T. F. Angew. Chem., Int. Ed. 2003, 42, 1364.
(b) Patel, S. J.; Jamison, T. F. Angew. Chem., Int. Ed. 2004, 43, 3941.
(c) Zhou, C. Y.; Zhu, S. F.; Wang, L. X., Zhou, Q. L. J. Am. Chem. Soc. 2010, 132, 10955.
(d) Holmes, M.; Schwartz, L. A.; Krische, M. J. Chem. Rev. 2018, 118, 6026.
[13] (a) Xie, Y. J.; Hu, J. H.; Wang, Y. Y.; Xia, C. G.; Huang, H. J. Am. Chem. Soc. 2012, 134, 20613.
(b) Liu, Y.; Xie, Y.; Wang, H.; Huang, H. J. Am. Chem. Soc. 2016, 138, 4314.
[14] (a) Bäckvall, J. E.; Nordberg, R. E.; Nyström, J. E.; Hoegberg, T.; Ulff, B. J. Org. Chem. 1981, 46, 3479.
(b) You, S. L.; Zhu, X. Z.; Luo, Y. M.; Hou, X. L.; Dai, L. X. J. Am. Chem. Soc. 2001, 123, 7471.
(c) Nagano, T.; Kobayashi, S. J. Am. Chem. Soc. 2009, 131, 4200.
(d) Xie, Y. J.; Hu, J. H.; Wang, Y. Y.; Xia, C.; Huang, H. J. Am. Chem. Soc. 2012, 134, 20613.
(e) Dubovyk, I.; Watson, I. D. G.; Yudin, A. K. J. Org. Chem. 2013, 78, 1559.
(f) Cai, A. J.; Guo, W. S.; Martínez-Rodríguez, L.; Kleij, A. W. J. Am. Chem. Soc. 2016, 138, 14194.
(g) Li, Y. G.; Li, L.; Yang, M. Y.; Kantchev, E. A. B. J. Org. Chem. 2017, 82, 4907.
[15] (a) Trost, B. M.; Zhang, T.; Sieber, J. D. Chem. Sci. 2010, 1, 427.
(b) Evans, P.; Grange, R.; Clizbe, E. Synthesis 2016, 48, 2911.
(c) Guo, W.; Cai, A.; Xie, J.; Kleij, A. W. Angew. Chem., Int. Ed. 2017, 56, 11797.
(d) Xia, C.; Shen, J.; Liu, D.; Zhang, W. Org. Lett. 2017, 19, 4251.
(e) Wang, Y. N.; Wang, B. C.; Zhang, M. M.; Gao, X. W.; Li, T. R.; Lu, L. Q.; Xiao, W. J. Org. Lett. 2017, 19, 4094.
[16] Yao, L.; Wang, C. J. Adv. Synth. Catal. 2015, 357, 384.
[17] Lu, B.; Feng, B.; Ye, H.; Chen, J. R.; Xiao, W. J. Org. Lett. 2018, 20, 3473.
[18] Ouyang, K.; Xi, Z. Acta Chim. Sinica 2013, 71, 13(in Chinese). (欧阳昆冰, 席振峰, 化学学报, 2013, 71, 13.)
/
| 〈 |
|
〉 |