

Chinese Journal of Organic Chemistry >
Sc(OTf)3 Catalyzed Oxo-Michael Addition to o-Quinone Methides by Alcohols
Received date: 2018-07-09
Revised date: 2018-08-26
Online published: 2018-09-10
Supported by
Project supported by the Shandong Provincial Natural Science Foundation (No. ZR2017BB033), the Youth Science Funds of Shandong Academy of Sciences (No. 2018QN0030) and the National Natural Science Foundation of China (No. 51503118).
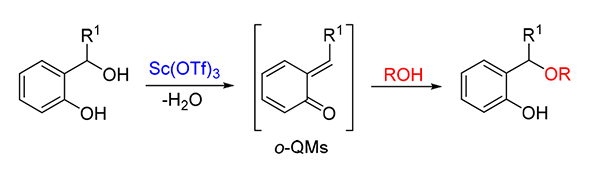
o-Quinone derivatives are not only a variety of active and important intermediate, but also widely used in the synthesis of natural products and medicinal chemistry. In the present study, the Sc(OTf)3 catalyzed oxo-Michael addition to o-quinone methides by alcohols was developed. The products were obtained in moderate to good yields (76%~97%) under mild conditions. Furthermore, the reaction could be scaled up to multigram scale.
Key words: o-quinone; scandium(III) triflate; oxo-Michael addition; green chemistry
Zhang Shuo , Peng Dan , Zhao Ning , Yu Yitao , Wang Feng , Liu Hailong , Yi Gang . Sc(OTf)3 Catalyzed Oxo-Michael Addition to o-Quinone Methides by Alcohols[J]. Chinese Journal of Organic Chemistry, 2019 , 39(2) : 555 -560 . DOI: 10.6023/cjoc201807017
[1] Pathak, T. P.; Sigman, M. S. J. Org. Chem. 2011, 76, 9210.
[2] Willis, N. J.; Bray, C. D. Chem.-Eur. J. 2012, 18, 9160.
[3] Caruana, L.; Fochi, M.; Bernardi, L. Molecules 2015, 20, 11733.
[4] Wang, Z.; Sun, J. Synthesis 2015, 47, 3629.
[5] Guo, C.; Song, J.; Luo, S.; Gong, L. Z. Angew. Chem., Int. Ed., 2010, 49, 5558.
[6] Van De Water, R, W.; Pettus, T. R. R. Tetrahedron 2002, 58, 5367.
[7] Kulikov, A.; Arumugam, S.; Popik, V. V. J. Org. Chem. 2008, 73, 7611.
[8] Mattson, A. E.; Scheidt, K. A. J. Am. Chem. Soc. 2007, 129, 4508.
[9] Luan, Y.; Schaus, S. E. J. Am. Chem. Soc. 2012, 134, 19965.
[10] Shaikh, A. K.; Cobb, A. J. A.; Varounis, G. Org. Lett. 2012, 14, 584.
[11] Chen, M. W.; Gao, L. L.; Ye, Z. S.; Jiang, G. F.; Zhou, Y. G. Chem. Commun. 2013, 49, 1660.
[12] Yoshida, H.; Watanabe, M.; Fukushima, H.; Ohshita, J.; Kunai, A. A. Org. Lett. 2004, 6, 4049.
[13] Bai, W. J.; David, J. G.; Feng, Z. G.; Weaver, M. G.; Wu, K. L.; Pettus, T. R. R. Acc. Chem. Res. 2014, 47, 3655.
[14] Caruana, L.; Fochi, M.; Bernardi, L. Molecules 2015, 20, 11733.
[15] Wang, Z.; Sun, J. Synthesis 2015, 47, 3629.
[16] Zhao, W.; Wang, Z.; Chu, B.; Sun, J. Angew. Chem., Int. Ed. 2015, 54, 1910.
[17] Huang, Y.; Hayashi, T. J. Am. Chem. Soc. 2015, 137, 7556.
[18] Wang, Z.; Ai, F.; Wang, Z.; Zhao, W.; Zhu, G.; Lin, Z.; Sun, J. J. Am. Chem. Soc. 2015, 137, 383.
[19] Wu, B.; Yu, Z.; Gao, X.; Lan, Y.; Zhou, Y.-G. Angew. Chem., Int. Ed. 2017, 56, 4006.
[20] Chen, P.; Wang, K. l; Guo, W.; Liu, X.; Liu, Y.; Li, C. Angew. Chem., Int. Ed. 2017, 56, 3689.
[21] Nising, C. F.; Brase, S. Chem. Soc. Rev. 2008, 37, 1218.
[22] Nising, C. F.; Brase, S. Chem. Soc. Rev. 2012, 41, 988.
[23] Heravi, M. M.; Hajiabbasi, P. Mol. Diversity 2014, 18, 411.
[24] Gu, Q.; Rong, Z.-Q.; Zheng, C.; You, S. L. J. Am. Chem. Soc. 2010, 132, 4056.
[25] Rubush, D. M.; Morges, M. A.; Rose, B. J.; Thamm, D. H.; Rovis, T. J. Am. Chem. Soc. 2012, 134, 13554.
[26] Shi, Y. L.; Shi, M. Org. Biomol. Chem. 2007, 5, 1499.
[27] Liang, M.; Zhang, S.; Jia, J.; Tung, C.-H.; Wang, J. W.; Xu, Z. H. Org. Lett. 2017, 19, 2526.
[28] Lai, Z. W.; Wang, Z. B.; Sun, J. W. Org. Lett. 2015, 17, 6058.
/
| 〈 |
|
〉 |