

Chinese Journal of Organic Chemistry >
Diastereoselective Construction of All-Carbon Quaternary Stereocenters via Intramolecular Oxidative Cross-Coupling Reaction
Received date: 2018-05-10
Revised date: 2018-09-13
Online published: 2018-09-26
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21772008, 21632002), the Shenzhen Science and Technology Project Program (No. GRCK2017042414425972), the Natural Science Foundation of Guangdong Province (No. 2016A030306011) and the Qingdao National Laboratory for Marine Science and Technology (No. LMDBKF201703).
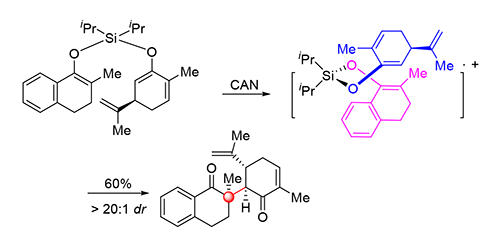
The formation of sterically hindered C—C bond represents a great challenge in modern synthetic organic chemistry. A particularly challenging issue is the construction of all-carbon quaternary stereocenters. Herein, a ceric ammonium nitrate (CAN)-mediated intramolecular oxidative cross-coupling of silyl ethers for direct construction of valuable polycyclic scaffolds is described. The reaction enables sterically congested vicinal all-carbon quaternary and tertiary stereocenters to be installed diastereoselectively. The developed method provides a concise and efficient approach for ligation of two different segments through a compact C—C bond formation, which has potential applications in the synthesis of complex molecules as well as sterically congested natural products.
Chen Wei , Guo Renyu , Gong Jianxian , Yang Zhen . Diastereoselective Construction of All-Carbon Quaternary Stereocenters via Intramolecular Oxidative Cross-Coupling Reaction[J]. Chinese Journal of Organic Chemistry, 2019 , 39(1) : 238 -248 . DOI: 10.6023/cjoc201805023
[1] For selected reviews, see:(a) Csákÿ, A. G.; Plumet, J. Chem. Soc. Rev. 2001, 30, 313.
(b) Yeung, C. S.; Dong, V. M. Chem. Rev. 2011, 111, 1215.
(c) Guo, F. H.; Clift, M. D.; Thomson, R. J. Eur. J. Org. Chem. 2012, 4881.
(d) Murarka, S.; Antonchick, A. P. Synthesis 2018, 50, 2150.
[2] Ivanoff, D.; Spassoff, A. Bull. Soc. Chim. Fr. 1935, 2, 76.
[3] For selected oxidative coupling examples, see:(a) Rathke, M. W.; Lindert, A. J. Am. Chem. Soc. 1971, 93, 4605.
(b) Ito, Y.; Konoike, T.; Saegusa, T. J. Am. Chem. Soc. 1975, 97, 649.
(c) Ito, Y.; Konoike, T.; Harada, T.; Saegusa, T. J. Am. Chem. Soc. 1977, 99, 1487.
(d) Frazier, Jr. R.; Harlow, R. J. Org. Chem. 1980, 45, 5408.
(e) Moeller, K. D.; Tinao, L. V. J. Am. Chem. Soc. 1992, 114, 1033.
(f) Mazzega, M.; Fabris, F.; Cossu, S.; Lucchi, O. D.; Lucchini, V.; Valle, G. Tetrahedron 1999, 55, 4427.
(g) Jang, H. Y.; Hong, J. B.; MacMillan, D. W. C. J. Am. Chem. Soc. 2007, 129, 7004.
(h) DeMartino, M. P.; Chen, K.; Baran, P. S. J. Am. Chem. Soc. 2008, 130, 11546.
(i) Casey, B. M.; Flowers, R. A. J. Am. Chem. Soc. 2011, 133, 11492.
(j) Amaya, T.; Maegawa, Y.; Masuda, T.; Osafune, Y.; Hirao. T. J. Am. Chem. Soc. 2015, 137, 10072.
(k) Kaiser, D.; Teskey, C. J.; Alder, P.; Maulide. N. J. Am. Chem. Soc. 2017, 139, 16040.
(l) Næsborg, L.; Corti, V.; Leth, L. A.; Poulsen, P. H.; Jørgensen. A. Angew. Chem., Int. Ed. 2018, 57, 1606.
(m) Tanaka, T.; Tanaka, T.; Tsuji, T.; Yazaki, R.; Ohshima, T. Org. Lett. 2018, 20, 3541.
[4] For selected applications of oxidative coupling reaction in natural product synthesis, see:(a) Baran, P. S.; Guerrero, C. A.; Ambhaikar, N. B.; Hafensteiner, B. D. Angew. Chem., Int. Ed. 2005, 44, 606.
(b) Baran, P. S.; Hafensteiner, B. D.; Ambhaikar, N. B.; Guerrero, C. A.; Gallagher, J. D. J. Am. Chem. Soc. 2006, 128, 8678.
(c) Martin, C. L.; Overman, L. E.; Rohde, J. M. J. Am. Chem. Soc. 2008, 130, 7568.
(d) Herzon, S. B.; Lu, L.; Woo, C. M.; Gholap, S. L. J. Am. Chem. Soc. 2011, 133, 7260.
(e) Konkol, L. C.; Guo, F. H.; Sarjeant, A. A.; Thomson, R. J. Angew. Chem., Int. Ed. 2011, 50, 9931.
(f) Jones, B. T.; Avetta, C. T.; Thomson, R. J. Chem. Sci. 2014, 5, 1794.
(g) You, L.; Liang, X.-T.; Xu, L.-M.; Wang, Y.-F.; Zhang, J.-J.; Su, Q.; Li, Y.-H.; Zhang, B.; Yang, S.-L.; Chen, J.-H.; Yang, Z. J. Am. Chem. Soc. 2015, 137, 10120.
(h) Robison, E. E.; Thomson, R. J. J. Am. Chem. Soc. 2018, 140, 1956.
[5] Wender, P. A.; Miller, B. L. In Organic Synthesis:Theory and Applications, Vol. 2, Ed.:Hudlicky, T., JAI Press, Greenwich, CT, 1993.
[6] (a) Tokuda, M.; Shigei, T.; Itoh, M. Chem. Lett. 1975, 621.
(b) Baciocchi, E.; Casu, A.; Ruzziconi, R. Tetrahedron Lett. 1989, 30, 3707.
[7] (a) Beeson, T. D.; Mastracchio, A.; Hong, J. B.; Ashton, K.; MacMillan, D. W. C. Science 2007, 316, 582.
(b) Yasu, Y.; Koike, T.; Akita, M. Chem. Commun. 2012, 48, 5355.
[8] (a) Schmittel, M.; Burghart, A.; Malisch, W.; Reising, J.; Söllner, R. J. Org. Chem. 1998, 63, 396.
(b) Schmittel, M.; Haeuseler, A. J. Organomet. Chem. 2002, 661, 169.
[9] (a) Avetta, C. T.; Konkol, L. C.; Taylor, C. N.; Dugan, K. C.; Stern, C. L.; Thomson, R. J. Org. Lett. 2008, 10, 5621.
(b) Konkol, L. C.; Jones, B. T.; Thomson, R. J. Org. Lett. 2009, 11, 5550.
[10] (a) Fuji, K. Chem. Rev. 1993, 93, 2037.
(b) Corey, E. J.; Guzman-Perez, A. Angew. Chem., Int. Ed. 1998, 37, 388.
(c) Christoffers, J.; Mann, A. Angew. Chem., Int. Ed. 2001, 40, 4591.
(d) Douglas, C. J.; Overman, L. E. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 5363.
(e) Christoffers, J.; Baro, A. Quaternary Stereocenters:Challenges and Solutions for Organic Synthesis, Wiley-VCH, Weinheim, 2005.
(f) Trost, B. M.; Jiang, C. Synthesis 2006, 369.
(g) Hawner. C.; Alexakis, A. Chem. Commun. 2010, 46, 7295.
(h) Shimizu, M. Angew. Chem., Int. Ed. 2011, 50, 5998.
(i) Hong, A. Y.; Stoltz, B. M. Eur. J. Org. Chem. 2013, 2745.
(j) Quasdorf, K. W.; Overman, L. E. Nature 2014, 516, 181.
(k) Long, R.; Huang, J.; Gong, J. X.; Yang, Z. Nat. Prod. Rep. 2015, 32, 1584.
[11] (a) Arya, P.; Joseph, R.; Chou, D. T. H. Chem. Biol. 2002, 9, 145.
(b) Juncosa Jr, J. I.; Hansen, M.; Bonner, L. A.; Cueva, J. P.; Maglathlin, R.; McCorvy, J. D.; Marona-Lewicka, D.; Lill, M. A.; Nichols, D. E. ACS Chem. Neurosci. 2013, 4, 96.
[12] (a) Narayanan, C. R.; Pachapurkar, R. V. Tetrahedron Lett. 1965, 48, 4333.
(b) Ziffer, H.; Weiss, U.; Narayanan, C. R.; Pachapurkar, R. V. J. Org. Chem. 1966, 31, 2691.
(c) Harris, M.; Henderson, R.; McCrindle, R.; Overton, K. H.; Turner, D. W. Tetrahedron 1968, 24, 1517.
[13] Fontana, A.; Muniain, C.; Cimino, G. J. Nat. Prod. 1998, 61, 1027.
[14] Gonzalez, M. Curr. Bioact. Compd. 2007, 3, 1.
[15] (a) Zhang, P. P.; Yan, Z. M.; Li, Y. H.; Gong, G. X.; Yang, Z. J. Am. Chem. Soc. 2017, 139, 13989.
(b) Huang, J.; Gu, Y. Q.; Guo, K.; Zhu, L.; Lan, Y.; Gong, G. X.; Yang, Z. Angew. Chem., Int. Ed. 2017, 56, 7890.
(c) Liu, D.-D.; Sun, T.-W.; Wang, K.-Y.; Lu, Y.; Zhang, S.-L.; Li, Y.-H.; Jiang, Y.-L.; Chen, J.-H.; Yang, Z. J. Am. Chem. Soc. 2017, 139, 5732.
(d) Huang, Z. H.; Huang, J.; Qu, Y. Z.; Zhang, W. B.; Gong, J. X.; Yang, Z. Angew. Chem., Int. Ed. 2018, 57, 8744.
[16] Paquette, L. A.; Bzowej, E. I.; Branan, B. M.; Stanton, K. J. J. Org. Chem. 1995, 60, 7277.
[17] (a) Dessau, R. M.; Heiba, E. I. J. Org. Chem. 1974, 39, 3457.
(b) Liu, X. G.; Chen, X. H.; Mohr, J. T. Org. Lett. 2016, 18, 3182.
[18] Kobayashi, Y.; Taguchi, T.; Tokuno, E. Tetrahedron Lett. 1977, 3741.
[19] CDCC 1501822(15), CDCC 1582641(30) and CDCC 1861685(32) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB21EZ, U. K.; fax:+44-1223336033.
[20] (a) Chatgilialoglu, C.; Studer, A. Encyclopedia of Radicals in Chemistry, Biology and Materials, Vols. 1~4, Wiley, Chichester, 2012.
(b) Zhang, N.; Samanta, S. R.; Rosen, B. M.; Percec, V. Chem. Rev. 2014, 114, 5848.
(c) Studer, A.; Curran, D. P. Angew. Chem., Int. Ed. 2016, 55, 58.
(d) Zard, S. Z. Org. Lett. 2017, 19, 1257.
[21] Frazier, Jr. R.; Harlow, R. J. Org. Chem. 1980, 45, 5408.
[22] (a) Schmittel, M.; Söllner, R. Chem. Ber./Rec. 1997, 130, 771.
(b) Schmittel, M.; Burghart, A.; Werner, H.; Laubender, M.; Söllner, R. J. Org. Chem. 1999, 104, 3077.
/
| 〈 |
|
〉 |