

Chinese Journal of Organic Chemistry >
Synthesis of Pyrazolo[1,2-b]phthalazine-5,10-diones Catalyzed by Poly(ethylene glycol) Grafted 4-Dimethylaminopyridine Functionalized Ionic Liquid
Received date: 2018-05-29
Revised date: 2018-07-09
Online published: 2018-10-20
Supported by
Project supported by the National Natural Science Foundation of China (No.21002050) the Key Project of Science and Technology of Henan Province (No. 182102310601) and the Key Scientific Research Projects of Henan Province (No. 17B150007).
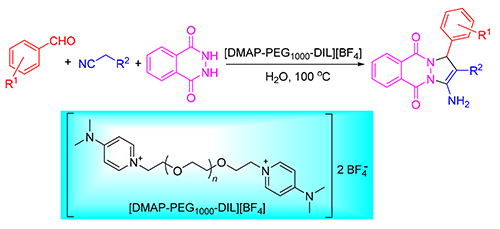
A novel poly(ethylene glycol) grafted 4-dimethylaminopyridine functionalized ionic liquid ([DMAP-PEG1000-DIL] [BF4]) was prepared and characterized by 1H NMR, 13C NMR, FT-IR and ESI-MS. In the presence of[DMAP-PEG1000-DIL] [BF4], various substituted aromatic aldehyde could react with malononitrile (or ethyl cyanoacetate) and phthalhydrazide in water to obtain a series of pyrazolo[1,2-b]phthalazine-5,10-dione derivatives with 85%~96% yields. The mild reaction conditions, high stable and recyclable ionic liquids, environmental friendliness are advantages of this methodology.
Wang Yinglei , Wan Zijuan , Luo Jun . Synthesis of Pyrazolo[1,2-b]phthalazine-5,10-diones Catalyzed by Poly(ethylene glycol) Grafted 4-Dimethylaminopyridine Functionalized Ionic Liquid[J]. Chinese Journal of Organic Chemistry, 2019 , 39(2) : 521 -526 . DOI: 10.6023/cjoc201805052
[1] (a) Ghahremanzadeh, R.; Shakibaei, G. I.; Bazgir, A. Synlett 2008, 1129.
(b) Sangani, C. B.; Makwana, J. A.; Duan, Y. T.; Thumar, N. J.; Zhao, M. Y.; Patel, Y. S.; Zhu, H. L. Res. Chem. Intermed. 2016, 42, 2101.
[2] Karthikeyan, G.; Pandurangan, A. J. Mol. Catal. A:Chem. 2012, 361, 58.
[3] Reddy, M. V.; Jeong, Y. T. Tetrahedron Lett. 2013, 54, 3546.
[4] Azarifar, A.; Nejat, Y. R.; Azarifar D. J. Iran. Chem. Soc. 2013, 10, 297.
[5] Reddy, M. V.; Kumar, P. C. R.; Reddy, G. C. S.; Reddy, C. S. C. R. Chim. 2014, 17, 1250.
[6] Ghorbani, V. R.; Noori, S.; Toghraei, S. Z.; Salimi, Z. RSC Adv. 2014, 4, 47925.
[7] Chaskar, A. Curr. Catal. 2014, 3, 266.
[8] Kidwai, M.; Chauhan, R. J. Heterocycl. Chem. 2014, 51, 1689.
[9] Vafaee, A.; Davoodnia, A.; Pordel. M.; Bozorgmehr, M. R. Orient. J. Chem. 2015, 31, 2153.
[10] Mulik, A. G.; Chandam, D. R.; Patil, D. R.; Patil, P. P.; Mulik, G. N.; Salunkhe, S. T.; Deshmukh, M. B. Res. Chem. Intermed. 2015, 41, 10085.
[11] Wang, W.; Li, C. H.; Yu, Y.; Li, X. J.; Guo, H. Y. J. Chem. Res. 2016, 40, 354.
[12] Tayade, Y. A.;·Dalal, D. S. Catal. Lett. 2017, 147, 1411.
[13] Tayebee, R.; Maleki, B.; Sabeti, M. J. Iran. Chem. Soc. 2017, 14, 1179.
[14] Piltan, M. Heterocycl. Commun. 2017, 23, 401.
[15] Sabour, F. H.; Nasr, E. M.; Mohammadpoor, B. I.; Tangesta-ninejad, S.; Moghadam, M.; Mirkhani, V. J. Iran. Chem. Soc. 2018, 15, 671.
[16] Raghuvanshi, D. S.; Singh, K. N. Tetrahedron Lett. 2011, 52, 5702.
[17] (a) Nabid, M. R.; Rezaei, S. J. T.; Ghahremanzadeh, R.; Bazgir, A. Ultrason. Sonochem. 2010, 17, 159.
(b) Kefayati, H.; Delafrooz, A.; Homayoon, S. Russ. J. Gen. Chem. 2016, 86, 1735.
[18] Kefayati, H.; Amlashi, S. H.; Kazemi, R. R.; Delafrooz, A. C. R. Chim. 2014, 17, 894.
[19] (a) Shaterian, H. R.; Mohammadnia, M. J. Mol. Liq. 2012, 173, 55.
(b) Mohamadpour, F.; Maghsoodlou, M. T.; Heydari, R.; Lashkari, M. Res. Chem. Intermed. 2016, 42, 7841.
[20] (a) Song, S. H.; Zhong, J.; He, Y. H.; Guan, Z. Tetrahedron Lett. 2012, 53, 7075.
(b) Pradhan, K.; Paul, S.; Das, R. A. Monatsh. Chem. 2014, 145, 1343.
(c) Mohamadpour, F.; Maghsoodlou, M. T.;·Heydari, R.; Lashkari, M. J. Iran. Chem. Soc. 2016, 13, 1549.
[21] (a) Colacino, E.; Martinez, J.; Lamaty, F.; Patrikeeva, L. S.; Khemchyan, L. L.; Ananikov, V. P.; Beletskaya, I. P. Coord. Chem. Rev. 2012, 256, 2893.
(b) Vafaeezadeh, M.; Hashemi, M. M. J. Mol. Liq. 2015, 207, 73.
[22] (a) Xu, H. T.; Zhang, C. H.; Chen, G.; Shen, R. P.; Ying, A. G. Chin. J. Org. Chem. 2016, 36, 2353(in Chinese). (徐慧婷, 张超怀, 陈钢, 沈润溥, 应安国, 有机化学, 2016, 36, 2353.)
(b) Zhang, W. T.; Sun, J.; Xu, F.; Zhu, H.; Yue, R. X.; Zhang, Y.; Niu, F. X. Chin. J. Org. Chem. 2017, 37, 3191(in Chinese). (张文婷, 孙健, 徐飞, 朱红, 岳瑞雪, 张毅, 钮福祥, 有机化学, 2017, 37, 3191.)
(c) Cen, J. H.; Yang, K.; Li, J. X.; Li, C.; Yang, S. R. Chin. J. Org. Chem. 2017, 37, 3213(in Chinese). (岑竞鹤, 杨凯, 李建晓, 李灿, 杨少容, 有机化学, 2017, 37, 3213.)
(d) Li, J. X.; Yang, S. R.; Wu, W. Q.; Jiang, H. F. Eur. J. Org. Chem. 2018, 1284.
[23] (a) Wu, C.; Peng, J. j.; Li, J. Y.; Bai, Y.; Hu, Y. Q.; Lai, G. Q. Catal. Commun. 2008, 10, 248.
(b) Cecchini, M. M.; Charnay, C.; Angelis, F. D.; Lamaty, F.; Martinez, J.; Colacino, E. ChemSusChem 2014, 7, 45.
(c) Xu, Y. S.; Zhang, F. X.; Li, J. Y.; Bai, Y.; Xiao, W. J.; Peng, J. J. Prog. Chem. 2015, 27, 1400(in Chinese). (徐艺凇, 张凤香, 厉嘉云, 白赢, 肖文军, 彭家建, 化学进展, 2015, 27, 1400.)
[24] (a) Wang, Y. L.; Luo, J.; Xing, T. T.; Liu, Z. L. Chin. J. Org. Chem. 2013, 33, 2016(in Chinese). (王英磊, 罗军, 邢昙昙, 刘祖亮, 有机化学, 2013, 33, 2016.)
(b) Wang, Y. L.; Yue, C.; Li, X.; Luo, J. C. R. Chim. 2016, 19, 1021.
(c) Wang, Y. L.; Liu, X. G.; Du, C. J.; Zhang, L. Synth. Commun. 2017, 47, 886.
/
| 〈 |
|
〉 |