

Chinese Journal of Organic Chemistry >
Chemoselective Synthesis of Substituted Benzoxazines and Imidazolidines by Reactions of Hydroxyl Substituted Ethylenediamine Derivatives with Aldehydes
Received date: 2018-08-06
Revised date: 2018-09-10
Online published: 2018-10-26
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21877034, 21372070) and the Scientific Research Fund of Hunan Provincial Education Department (No. 17A066).
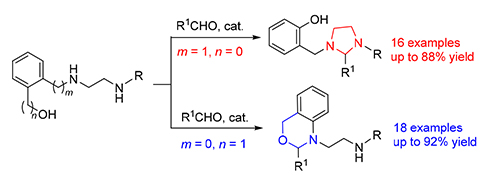
Lewis acid La(OTf) 3-catalyzed chemoselective cyclization of hydroxyl substituted ethylenediamine derivatives with aldehydes has been described for the first time, which provides efficient access to diversely functionalized 1,3-imidazoli-dines and 3,1-benzoxazines in generally good yields only by adjusting the position of the methylene group within hydroxyl substituted ethylenediamines. The reaction is suitable to aromatic aldehydes and aliphatic ones. Plausible mechanisms are also proposed to explain the observed reaction modes, wherein the nucleophilicity of nitrogen and oxygen atoms plays an important role in controlling the chemoselectivity.
Tang Zilong , Wang Ming , Yao Yuan , Tan Jingzhao , Dai Ningning , Li Xinxing , Peng Lifen , Jiao Yinchun . Chemoselective Synthesis of Substituted Benzoxazines and Imidazolidines by Reactions of Hydroxyl Substituted Ethylenediamine Derivatives with Aldehydes[J]. Chinese Journal of Organic Chemistry, 2019 , 39(3) : 800 -810 . DOI: 10.6023/cjoc201808003
[1] Neuvonen, K.; Pihlaja, K. J. Chem. Soc., Perkin Trans 2 1988, 461.
[2] Tang, Z.; Chen, W.; Zhu, Z.; Liu, H. J. Heterocycl. Chem. 2011, 48, 255.
[3] Mangion, K.; Chen, C.; Li, H.; Maligres, P.; Chen, Y.; Christensen, M.; Cohen, R.; Jeon, I.; Klapars, A.; Krska, S.; Nguyen, H.; Reamer, R. A.; Sherry, B. D.; Zavialov, I. Org. Lett. 2014, 16, 2310.
[4] Tang, Z.; Chen, W.; Zhu, Z.; Liu, H. Synth. Commun. 2012, 42, 1372.
[5] Mathew, B. P.; Nath, M. J. Heterocycl. Chem. 2009, 46, 1003.
[6] Colin, J. L.; Loubinoux, B. Tetrahedron Lett. 1982, 23, 4245.
[7] Richers, M. T.; Breugst, M.; Platonova, A. Y.; Ullrich, A.; Dieckmann, A.; Houk, K. N.; Seidel, D. J. Am. Chem. Soc. 2014, 136, 6123.
[8] Xu, F.; Qian, X.-Y.; Li, Y.-J.; Xu, H.-C. Org. Lett. 2017, 19, 6332.
[9] Chen, X.; Hao, W.; Liu, Y. Org. Biomol. Chem. 2017, 15, 3423.
[10] Garg, V.; Kumar, A.; Chaudhary, A.; Agrawal, S.; Tomar, P.; Sreenivasan, K. K. Med. Chem. Res. 2013; 22, 5256.
[11] Chen, Y.; Cass, S. L.; Kutty, S. K.; Yee, E. M. H.; Chan, D. S. H.; Gardner, C. R.; Vittorio, O.; Pasquier, E.; Black, D. S.; Kumar, N.; Bioorg. Med. Chem. Lett. 2015, 25, 5377.
[12] Morrison, R.; Al-Rawi, J. M. A.; Jennings, I.; Thompson, P. E. Eur. J. Med. Chem. 2016, 110, 326.
[13] Ihmaid, S. K.; Ai-Rawi, J. M. A.; Bradley, C. J.; Angove, M. J.; Robertson, M. N. Eur. J. Med. Chem. 2012, 57, 85.
[14] Nemecek. P.; Mocak, J.; Lehotay, J.; Waisser, K. Chem. Papers 2013, 67, 305.
[15] Pastemak, A.; Goble, S. D.; Struters, M.; Vicario, P. P.; Ayala, J. M.; Salvo, J. D.; Kilbum, R.; Wisniewski, T.; Demartino, J. A.; Mills, S. G.; Yang, L. ACS Med. Chem. Lett. 2010, 1, 14.
[16] Tong, L.; Yu, W.; Chen, L.; Selyutin, O.; Dwyer, M. P.; Nair, A. G.; Mazzola, R.; Kim, J.; Sha, D.; Yin, J.; Ruck, R. T.; Davies, I. W.; Hu, B.; Zhong B.; Hao, J.; Ji, T.; Zan, S.; Liu, R.; Agrawal, S.; Xia, E.; Curry, S.; McMonagle, P.; Bystol, K.; Lahser, F.; Carr, D.; Rokosz, L.; Ingravallo, P.; Chen, S.; Feng, K.; Cartwright, M.; Asante-Appiah, E.; Kozlowski, J. A. J. Med. Chem. 2017, 60, 290.
[17] Ihmaid, S.; Ahmed, H. E. A.; Ali, A. A.; Sherif, Y. E.; Tarazi, H. M.; Riyadh, S. M.; Zayed, M. F.; Abulkhair, H. S.; Rateb, H. S. Bioorg. Chem. 2017, 72, 234.
[18] Sharma, V.; Amarnath, N.; Shukla, S.; Ayana, R.; Kumar, N.; Yadav, N.; Kannan, D.; Sehrawat, S.; Pati, S.; Lochab, B.; Singh, S.; Bioorg. Med. Chem. Lett. 2018, 28, 1629.
[19] Zhang, P.; Terefenko, E. A.; Fensome, A.; Zhang, Z.; Zhu, Y.; Cohen, J.; Winneker, R.; Wrobel, J.; Yardley, J. Bioorg. Med. Chem. Lett. 2002, 12, 787.
[20] Nguyen, T. T.; Amey, R. L.; Martin, J. C. J. Org. Chem. 1982, 47, 1024.
[21] Kobzina, J. W.; Creek, W. US 4030906, 1977.
[22] Sugiyama, H.; Hosoda, K.; Kumagai, Y.; Takeuchi, M.; Okada, M. U. US 4596801, 1986.
[23] Charmantray, F.; Demeunynck, M.; Carrez, D.; Croisy, A.; Lansiaux, A.; Bailly, C.; Colson, P. J. Med. Chem. 2003, 46, 967.
[24] Badolato, M.; Carullo, G.; Armentano, B.; Panza, S.; Malivindi, R. Bioorg. Med. Chem. Lett. 2017, 27, 3092.
[25] Dias, N.; Goossens, J.; Baldeyrou, B.; Lansiaux, A.; Colson, P.; Di Salvo, A.; Bernal, J.; Turnbull, A.; Mincher, D. J.; Bailly, C. Bioconjugate Chem. 2005, 16, 949.
[26] Marasini, B. P.; Rahim, F.; Perveen, S.; Karim, A.; Khan, K. M.; Rahman, A.; Choudhary, M. L. Bioorg. Chem. 2017, 70, 210.
[27] Braga, A. L.; Vargas, F.; Silveira, C. C.; de Andrade, L. H. Tetrahedron Lett. 2002, 43, 2335.
[28] Lee, E.; Kim, S.; Jung, B.; Ahn, W.; Kim, G. Tetrahedron Lett. 2003, 44, 1971.
[29] Arai, T.; Mishiro, A.; Yokoyama, N.; Suzuki, K.; Sato, H. J. Am. Chem. Soc. 2010, 132, 5338.
[30] Wang, Y.; Zhang, H.; Li, C.; Fan, T.; Shi, F. Chem. Commun. 2016, 52, 1804.
[31] Matosiuk, D.; Fidecka, S.; Antkiewicz-Michaluk, L.; Dybala, I.; Koziol, A. E. Eur. J. Med. Chem. 2001, 36, 783.
[32] Byrtus, H.; Obniska, J.; Czopek, A.; Kaminski, K.; Pawlowski, M. Bioorg. Med. Chem. 2011, 19, 6149.
[33] Kim, M.; Kim, H.; Kim, H.; Chin, J. J. Org. Chem. 2017, 82, 12050.
[34] Chen, J. R.; Hu, X. Q.; Lu, L. Q.; Xiao, W. J. Chem. Rev. 2015, 115, 5301.
[35] Chen, D. Z.; Xiao, W. J.; Chen, J. R. Org. Chem. Front. 2017, 4, 1289.
[36] Tang, Z.; Tan, J.; Cai, L.; Li, X.; Liu, W. Chin. J. Appl. Chem. 2017, 34, 345.
[37] CCDC 1823294 for compound 5ba, see the Supporting Information for detail.
[38] Tang, Z.; Wang, L.; Tan, J.; Yao, Y.; Peng, L. Chin. J. Appl. Chem. 2018 35, 1190.
/
| 〈 |
|
〉 |