

Chinese Journal of Organic Chemistry >
Design, Synthesis and Evaluation of Novel Gd-Based 1,4,7,10-Tetraazacyclododecan-N,N',N,N'-tetraacetic Acid-Hydrazide Derived Contrast Agents for Magnetic Resonance Imaging
Received date: 2018-08-07
Revised date: 2018-09-30
Online published: 2018-10-26
Supported by
Project supported by the Natural Science Foundation of Jiangsu Province (No. BK20181486), the Natural Science Foundation of the Jiangsu Higher Education Institutions (Nos. 17KJB320001, 16KJB540006), the Overseas Training Program for Excellent Young Teachers and Principals of Jiangsu Province and the Qing Lan Project of Jiangsu Province.
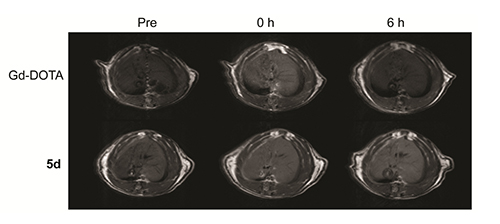
Magnetic resonance imaging (MRI) is widely used in diagnostic medicine and soft tissue imaging. Contrast agents (CAs) can improve the specificity of MRI enhancement. Herein, the design, synthesis and characterization of twelve novel Gd-based 1,4,7,10-tetraazacyclododecan-N,N',N,N'-tetraacetic acid (DOTA) -hydrazide derived contrast agents for MRI were reported. Among of them, 5d, 5h and 5l exhibit higher longitudinal relaxivities than the clinical Gd-DOTA at 0.5 T. The relaxivities r1 of 5d, 5h and 5l are 4.67, 4.85 and 5.33 L·mmol-1·s-1 respectively. In vivo liver-target MRI shows that the potential of complex 5d was used as a novel liver-target contrast agent for MRI.
Sun Hongshun , Zhou Jin , Li Yulong , Jiang Hong , Zhang Yan , Wang Jianqiang , Guo Cheng , Shen Linjiang . Design, Synthesis and Evaluation of Novel Gd-Based 1,4,7,10-Tetraazacyclododecan-N,N',N,N'-tetraacetic Acid-Hydrazide Derived Contrast Agents for Magnetic Resonance Imaging[J]. Chinese Journal of Organic Chemistry, 2019 , 39(3) : 778 -785 . DOI: 10.6023/cjoc201808005
[1] Merbach, A.-E.; Helm, L.; Toth, E. The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging, Wiley, Chichester, 2013, Preface.
[2] He, W.; Oliver, T.-B.; Michael, G.-K.; Eric, C.-H.; Mariya, B.; Agata, W.; Ou, C.; Yue, C.; Nan, L.; Satoshi, O.; Jose, M. C.; Markus, H.; Christian, T.-F.; Daniel, M.-M.; Gerhard, A.; Harald, I.; Alan, J.; Peter, N.; Moungi, G.-B. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 2325.
[3] Carr, D.-H.; Brown, J.; Bydder, G.-M.; Steiner, R.-E.; Weinmann, H.-J.; Speck, U.; Hall, A.-S.; Young, I.-R. Am. J. Roentgenol. 1984, 143, 215.
[4] Lohrke, J.; Frenzel, T.; Endrikat, J.; Alves, F.-C.; Grist, T.-M.; Law, M.; Lee, J.-M.; Leiner, T.; Li, K.-C.; Nikolaou, K.; Prince, M.-R.; Schild, H.-H.; Weinreb, J.-C.; Yoshikawa, K.; Pietsch, H. Adv. Ther. 2016, 33, 1.
[5] Pierre, V.-C.; Allen, M.-J.; Caravan, P. J. Biol. Inorg. Chem. 2014, 19, 127.
[6] Hermann, P.; Kotek, J.; Kubicek, V.; Lukes, I. Dalton Trans. 2008, 23, 3027.
[7] Thian, Y.-L.; Riddell, A.-M.; Koh, D.-M. Cancer Imaging 2013, 13, 567.
[8] Huckle, J.-E.; Altun, E.; Jay, M.-J.; Semelka, R.-C. Invest. Radiol. 2016, 51, 236.
[9] McMahon, M.-T.; Chan, K.-W. Adv. Cancer Res. 2014, 124, 297.
[10] Frullano, L.; Zhu, J.; Miller, R.-H.; Wang, Y.-J. Med. Chem. 2013, 56, 1629.
[11] Song, Y.; Kohlmeir, E.-K.; Meade, T.-J. J. Am. Chem. Soc. 2008, 130, 6662.
[12] Zhou, Y.; Kim, Y.-S.; Yan, X.; Jacobson, O.; Chen, X.; Liu, S. Mol. Pharm. 2011, 8, 1198.
[13] Barge, A.; Tei, L.; Upadhyaya, D.; Fedeli, F.; Beltrami, L.; Stefania, R.; Aime, S.; Cravotto, G. Org. Biomol. Chem. 2008, 6, 1176.
[14] Wang, S.-C.; Hsu, Y.-S.; Hsiao, C.-T.; Wu, C.-C.; Sue, Y.-C.; Alshehri, S.-M.; Ahamad, T.; Yamauchi, Y.; Chen, J.-E.; Wu, K.-C.-W.; Shieh, F.-K. J. Inorg. Organomet. Polym. 2016, 26, 165.
[15] Zhang, W.; Lu, C.; Zhao, G.; Zhang, J.; Fang, X.; Wang, P.; Fang, X.; Xu, J.; Yang, W. Z. Anorg. Allg. Chem. 2015, 641, 578.
[16] Cresens, E.; Ni, Y.-C.; Adriaens, P.; Verbruggen, A.; Marchal, G. WO 2002038546, 2002[Chem. Abstr. 2002, 136, 379071].
[17] Zhang, J.-D.; Curry, K. WO 2016090491, 2016[Chem. Abstr. 2016, 165, 112684].
[18] Frullano, L.; Tejerina, B.; Meade, T.-J. Inorg. Chem. 2006, 45, 8489.
[19] Zhou, J.; Yang, H.-D.; Sun, H.-S.; Li, Y.-L.; Jiang, H. Chin. J. Synth. Chem. 2017, 25, 159(in Chinese). (周进, 杨海东, 孙宏顺, 李玉龙, 蒋蕻, 合成化学, 2017, 25, 159.)
[20] Sun, H.-S.; Li, Y.-L.; Jiang, H.; Guo, C.; Shen, L.-J. Chin. J. Org. Chem. 2018, 38, 1779(in Chinese). (孙宏顺, 李玉龙, 蒋蕻, 郭成, 沈临江, 有机化学, 2018, 38, 1779.)
/
| 〈 |
|
〉 |