

Chinese Journal of Organic Chemistry >
Copper-Catalyzed Hydroxytrifluoromethylthiolation of Arylpropynones
Received date: 2018-08-30
Revised date: 2018-10-23
Online published: 2018-11-12
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21332010, 21421002), the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB20000000), and the Youth Innovation Promotion Association of the Chinese Academy of Sciences (No. 2016234).
Recently, the preparation of fluorinated compounds through difunctionalization strategies has become a hot research area in fluorine chemistry. In this work, a copper-catalyzed hydroxytrifluoromethylthiolation of arylpropynones for the synthesis of the corresponding trifluoromethylthiolated enols was developed. The copper salt and solvent are crucial to the yields of this reaction. Under optimized reaction conditions, a series of trifluoromethylthiolated enols were obtained in moderate to good yields.
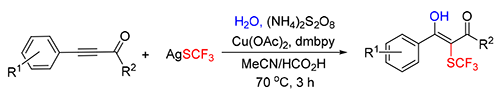
Key words: copper; trifluoromethylthiolation; hydroxylation; propynone; radical
Hu Juanjuan , Huang Yangen , Xu Xiuhua , Qing Fengling . Copper-Catalyzed Hydroxytrifluoromethylthiolation of Arylpropynones[J]. Chinese Journal of Organic Chemistry, 2019 , 39(1) : 177 -182 . DOI: 10.6023/cjoc201808041
[1] (a) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320.
(b) Meanwell, N. A. J. Med. Chem. 2011, 54, 2529.
(c) Cametti, M.; Crousse, B.; Metrangolo, P.; Milani, R.; Resnati, G. Chem. Soc. Rev. 2012, 41, 31.
(d) Wang, J.; Sánchez-Roselló, M.; Aceña, J. L.; del Pozo, C.; Sorochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Liu, H. Chem. Rev. 2014, 114, 2432.
(e) Gouverneur, V.; Seppelt, K. Chem. Rev. 2015, 115, 563.
(f) Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Aceña, J. L.; Soloshonok, V. A.; Izawa, K.; Liu, H. Chem. Rev. 2016, 116, 422.
(g) Rong, J.; Ni, C.; Wang, Y.; Kuang, C.; Gu, Y.; Hu, J. Acta Chim. Sinica 2017, 75, 105(in Chinese). (荣健, 倪传法, 王云泽, 匡翠文, 顾玉诚, 胡金波, 化学学报, 2017, 75, 105.)
(h) Meanwell, N. A. J. Med. Chem. 2018, 61, 5822.
[2] (a) Hansch, C.; Leo, A.; Unger, S. H.; Kim, K. H.; Nikaitani, D.; Lien, E. J. J. Med. Chem. 1973, 16, 1207.
(b) Hansch, C.; Leo, A.; Taft, R. W. Chem. Rev. 1991, 91, 165.
[3] (a) Chu, L.; Qing, F.-L. Acc. Chem. Res. 2014, 47, 1513.
(b) Toulgoat, F.; Alazet, S.; Billard, T. Eur. J. Org. Chem. 2014, 2415.
(c) Shao, X.; Xu, C.; Lu, L.; Shen, Q. Acc. Chem. Res. 2015, 48, 1227.
(d) Xu, H.-H.; Matsuzaki, K.; Shibata, N. Chem. Rev. 2015, 115, 731.
(e) Zhang, K.; Xu, X.-H.; Qing, F.-L. Chin. J. Org. Chem. 2015, 35, 556(in Chinese). (张珂, 徐修华, 卿凤翎, 有机化学, 2015, 35, 556.)
(f) Chachignon, H.; Cahard, D. Chin. J. Chem. 2016, 34, 445.
(g) Barata-Vallejo, S.; Bonesi, S.; Postigo, A. Org. Biomol. Chem. 2016, 14, 7150.
(h) Zheng, H.; Huang, Y.; Weng, Z. Tetrahedron Lett. 2016, 57, 1397.
[4] (a) Sheng, J.; Li, S.; Wu, J. Chem. Commun. 2014, 50, 578.
(b) Zhang, K.; Liu, J.-B.; Qing, F.-L. Chem. Commun. 2014, 50, 14157.
(c) Zhu, L.; Wang, G.; Guo, Q.; Xu, Z.; Zhang, D.; Wang, R. Org. Lett. 2014, 16, 5390.
(d) Yang, T.; Lu, L.; Shen, Q. Chem. Commun. 2015, 51, 5479.
(e) Chen, D.-Q.; Gao, P.; Zhou, P.-X.; Song, X.-R.; Qiu, Y.-F.; Liu, X.-Y.; Liang, Y. M. Chem. Commun. 2015, 51, 6637.
(f) Fuentes, N.; Kong, W.; Fernández-Sánchez, L.; Merino, E.; Nevado, C. J. Am. Chem. Soc. 2015, 137, 964.
(g) Luo, J.; Zhu, Z.; Liu, Y.; Zhao, X. Org. Lett. 2015, 17, 3620.
(h) Xu, C.; Shen, Q. Org. Lett. 2015, 17, 4561.
(i) Liu, X.; An, R.; Zhang, X.; Luo, J.; Zhao, X. Angew. Chem., Int. Ed. 2016, 55, 5846.
(j) Zhang, P.; Li, M.; Xue, X.-S.; Xu, C.; Zhao, Q.; Liu, Y.; Wang, H.; Guo, Y.; Lu, L.; Shen, Q. J. Org. Chem. 2016, 81, 7486.
(k) Jin, D.-P.; Gao, P.; Chen, D.-Q.; Chen, S.; Wang, J.; Liu, X.-Y.; Liang, Y.-M. Org. Lett. 2016, 18, 3486.
(l) Luo, J.; Liu, Y.; Zhao, X. Org. Lett. 2017, 19, 3434.
(m) Pan, S.; Huang, Y.; Xu, X.-H.; Qing, F.-L. Org. Lett. 2017, 19, 4624.
(n) Liu, X.; Liang, Y.; Ji, J.; Luo, J.; Zhao, X. J. Am. Chem. Soc. 2018, 140, 4782.
(o) Xiao, Z.; Liu, Y.; Zheng, L.; Liu, C.; Guo, Y.; Chen, Q.-Y. J. Org. Chem. 2018, 83, 5836.
(p) Xi, C.-C.; Chen, Z.-M.; Zhang, S.-Y.; Tu, Y.-Q. Org. Lett. 2018, 20, 4227.
[5] Wu, W.; Dai, W.; Ji, X.; Cao, S. Org. Lett. 2016, 18, 2918.
[6] (a) Guo, C.-H.; Chen, D.-Q.; Chen, S.; Liu, X.-Y. Adv. Synth. Catal. 2017, 359, 2901.
(b) Li, H.; Liu, S.; Huang, Y.; Xu, X.-H.; Qing, F.-L. Chem. Commun. 2017, 53, 10136.
[7] (a) Tlili, A.; Alazet, S.; Glenadel, Q.; Billard, T. Chem.-Eur. J. 2016, 22, 10230.
(b) Pan, S.; Li, H.; Huang, Y.; Xu, X.-H.; Qing, F.-L. Org. Lett. 2017, 19, 3247.
[8] Jiang, L.; Ding, T.; Yi, W.-B.; Zeng, X.; Zhang, W. Org. Lett. 2018, 20, 2236.
[9] (a) Ferry, A.; Billard, T.; Langlois, B. R.; Bacqué, E. Angew. Chem., Int. Ed. 2009, 48, 8551.
(b) Wu, J.-J.; Xu, J.; Zhao, X. Chem.-Eur. J. 2016, 22, 15265.
[10] (a) Yang, Y.; Jiang, X.-L.; Qing, F.-L. J. Org. Chem. 2012, 77, 7538.
(b) Wu, X.; Chu, L.; Qing, F.-L. Angew. Chem. Int. Ed. 2013, 52, 2198.
(c) Jiang, X.-Y.; Qing, F.-L. Angew. Chem. Int. Ed. 2013, 52, 14177.
(d) Yu, W.; Xu, X.-H.; Qing, F.-L. Adv. Synth. Catal. 2015, 357, 2039.
(e) Yang, B.; Xu, X.-H.; Qing, F.-L. Org. Lett. 2015, 17, 1906.
(f) Lin, Q.-Y.; Xu, X.-H.; Zhang, K.; Qing, F.-L. Angew. Chem., Int. Ed. 2016, 55, 1479.
(g) Yang, B.; Xu, X.-H.; Qing, F.-L. Chin. J. Chem. 2016, 34, 465.
(h) Lin, Q.-Y.; Ran, Y.; Xu, X.-H.; Qing, F.-L. Org. Lett. 2016, 18, 2419.
(i) Yu, W.; Xu, X.-H.; Qing, F.-L. Org. Lett. 2016, 18, 5130.
(j) Yang, B.; Xu, X.-H.; Qing, F.-L. Org. Lett. 2016, 18, 5956.
(k) Yang, B.; Yu, D.; Xu, X.-H.; Qing, F.-L. ACS Catal. 2018, 8, 2839.
(l) Ouyang, Y.; Xu, X.-H.; Qing, F.-L. Angew. Chem., Int. Ed. 2018, 57, 6926.
[11] (a) Yin, F.; Wang, X.-S. Org. Lett. 2014, 16, 1128.
(b) Guo, S.; Zhang, X.; Tang, P. Angew. Chem., Int. Ed. 2015, 54, 4065.
(c) Wu, H.; Xiao, Z.; Wu, J.; Guo, Y.; Xiao, J.-C.; Liu, C.; Chen, Q.-Y. Angew. Chem., Int. Ed. 2015, 54, 4070.
(d) Qiu, Y.-F.; Zhu, X.-Y.; Li, Y.-X.; He, Y.-T.; Yang, F.; Wang, J.; Hua, H.-L.; Zheng, L.; Wang, L.-C.; Liu, X.-Y.; Liang, Y.-M. Org. Lett. 2015, 17, 3694.
(e) Zeng, Y.-F.; Tan, D.-H.; Chen, Y.; Lv, W.-X.; Liu, X.-G.; Li, Q.; Wang, H. Org. Chem. Front. 2015, 2, 1511.
(f) Li, C.; Zhang, K.; Xu, X.-H.; Qing, F.-L. Tetrahedron Lett. 2015, 56, 6273.
(g) Pan, S.; Huang, Y.; Qing, F. L. Chem. Asian J. 2016, 11, 2854.
(h) Huang, F.-Q.; Wang, Y.-W.; Sun, J.-G.; Xie, J.; Qi, L.-W.; Zhang, B. RSC Adv. 2016, 6, 52710.
(i) Song, Y.-K.; Qian, P.-C.; Chen, F.; Deng, C.-L.; Zhang, X.-G. Tetrahedron 2016, 72, 7589.
(j) Ji, M. S.; Wu, Z.; Yu, J. J.; Wan, X. B.; Zhu, C. Adv. Synth. Catal. 2017, 359, 1959.
(k) He, B.; Xiao, Z.; Wu, H.; Guo, Y.; Chen, Q.-Y.; Liu, C. RSC Adv. 2017, 7, 880.
(l) Liu, K.; Jin, Q.; Chen, S.; Liu, P. N. RSC Adv. 2017, 7, 1546.
(m) Li, M.; Petersen, J. L.; Hoover, J. M. Org. Lett. 2017, 19, 638.
(n) Cheng, Z.-F.; Tao, T.-T.; Feng, Y.-S.; Tang, W.-K.; Xu, J.; Dai, J.-J.; Xu, H.-J. J. Org. Chem. 2018, 83, 499.
[12] (a) Ye, Y.; Sanford, M. S. J. Am. Chem. Soc. 2012, 134, 9034.
(b) Huang, L.; Zheng, S.-C.; Tan, B.; Liu, X.-Y. Chem.-Eur. J. 2015, 18, 6718.
(c) Yu, L.-Z.; Wei, Y.; Shi, M. Chem. Commun. 2016, 52, 13163.
(d) Cheng, C.; Liu, S.; Lu, D.; Zhu, G. Org. Lett. 2016, 18, 2852.
[13] (a) Wu, W.; Zhang, X.; Liang, F.; Cao, S. Org. Biomol. Chem. 2015, 13, 6992.
(b) Zhang, J.; Yang, J.-D.; Zheng, H.; Xue, X.-S.; Mayr, H.; Cheng, J.-P. Angew. Chem. Int. Ed. 2018, 57, 12690.
/
| 〈 |
|
〉 |