

Chinese Journal of Organic Chemistry >
Synthesis and Evaluation of Chalcone Derivatives as Novel Anticancer Agents
Received date: 2018-08-29
Revised date: 2018-10-23
Online published: 2018-11-26
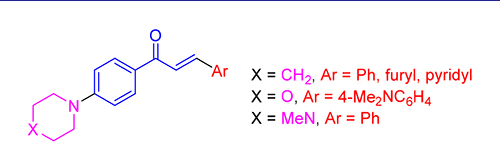
Three series of chalcones bearing a piperidino, morpholino, and 1-methylpiperazino moiety were synthesized in two steps with the key step being Claisen-Schmidt condensation and tested for the activity against five cell lines, MCF-7 (human breast adenocarcinoma cell line), A549 (human lung adenocarcinoma epithelial cell line), HL-60 (human leukemia cell line), Hela (human cervical cancer cell line), and Bewo (human chorionic tumor cell line) by thiazolyl blue tetrazolium bromide (MTT) assay. Some chalcones exhibited good anticancer activity, and among them 4a, 4e, 4f, 4j, 4m, and 4o displayed the best anticancer activity for MCF-7 breast cancer cells, A549 lung cancer cells, and HL-60 leukemia cancer cells with IC50 values below 10 μmol/L, respectively.
Key words: chalcone; condensation; anticancer agents; MTT assay
Sheng Qiwei , Zhao Wanqiu , Zeng Ming , Xie Zhongpao , Xia Yaping , Cui Dongmei . Synthesis and Evaluation of Chalcone Derivatives as Novel Anticancer Agents[J]. Chinese Journal of Organic Chemistry, 2019 , 39(3) : 703 -708 . DOI: 10.6023/cjoc201808037
[1] Lahtchev, K. L.; Batovska, D. I.; Parushev, S. P.; Ubiyvovk, V. M.; Sibirny, A. A. Eur. J. Med. Chem. 2008, 43, 2220.
[2] (a) Kantevari, S.; Addla, D.; Bagul, P. K.; Sridhar, B.; Banerjee, S. K. Bioorg. Med. Chem. 2011, 19, 4772.
(b) Yan, Y. K.; Xu, Q.; Gao, Y.; Liu, H.; Tang, X. R. Chin. J. Org. Chem. 2018, 38, 1763(in Chinese). (严映坤, 徐侨, 高扬, 刘辉, 唐孝荣, 有机化学, 2018, 38, 1763.)
[3] (a) Cho, S.; Kim, S.; Jin, Z.; Yang, H.; Han, D.; Baek, N. I.; Jo, J.; Cho, C. W.; Park, J. H.; Shimizu, M.; Jin, Y. H. Res. Commun. 2011, 413, 637.
(b) Zhang, E.; Wang, M. M.; Xu, S. M.; Wang, S.; Zhao, D.; Bai, P. Y.; Cui, D. Y.; Hua, Y. G.; Wang, Y. N.; Qin, S. S; Liu, H. M. Chin. J. Org. Chem. 2017, 37, 959(in Chinese). (张恩, 王铭铭, 徐帅民, 王上, 赵娣, 白鹏燕, 崔得运, 化永刚, 王亚娜, 秦上尚, 刘宏民, 有机化学, 2017, 37, 959.)
[4] Sato, Y.; He, J.; Nagai, H.; Tani, T.; Akao, T. Biol. Pharm. Bull. 2007, 30, 145.
[5] Luo, Y.; Song, R.; Li, Y.; Zhang, S.; Liu, Z. J.; Fu, J.; Zhu, H. L. Bioorg. Med. Chem. Lett. 2012, 22, 3039.
[6] Zhao, L.; Jin, H.; Sun, L.; Piao, H.; Quan, Z. Bioorg. Med. Chem. Lett. 2005, 15, 5027.
[7] Mahapatra, D. K.; Asati, V.; Bharti, S. K. Eur. J. Med. Chem. 2015, 92, 839.
[8] Mascarello, A.; Chiaradia, L. D.; Vernal, J.; Villarino, A.; Guido, R. V.; Perizzolo, P.; Poirier, V.; Wong, D.; Martins, P. G.; Nunes, R. J.; Yunes, R. A.; Andricopulo, A. D.; Av-Gay, Y.; Terenzi, H. Bioorg. Med. Chem. 2010, 18, 3783.
[9] Mahapatra, D. K.; Bharti, S. K.; Asati, V. Eur. J. Med. Chem. 2015, 98, 69.
[10] Lee, Y. S.; Lim, S. S.; Shin, K. H.; Kim, Y. S.; Ohuchi, K.; Jung, S. H. Biol. Pharm. Bull. 2006, 29, 1028.
[11] Aoki, N.; Muko, M.; Ohta, E.; Ohta, S. J. Nat. Prod. 2008, 71, 1308.
[12] Sashidhara, K. V.; Palnati, G. R.; Sonkar, R.; Avula, S. R.; Awasthi, C.; Bhatia, G. Eur. J. Med. Chem. 2013, 64, 422.
[13] Sashidhara, K. V.; Rao, K. B.; Kushwaha, V.; Modukuri, R. K.; Verma, R.; Murthy, P. K. Eur. J. Med. Chem. 2014, 81, 473.
[14] Rizvi, S. U. F.; Siddiqui, H. L.; Johns, M.; Detorio, M.; Schinazi, R. F. Med. Chem. Res. 2012, 21, 3741.
[15] Tomar, V.; Bhattacharjee, G.; Kamaluddin; Rajakumar, S.; Srivastava, K.; Puri, S. K. Eur. J. Med. Chem. 2010, 45, 745.
[16] Abdullah, M. I.; Mahmood, A.; Madni, M.; Masood, S.; Kashif, M. Bioorg. Chem. 2014, 54, 31.
[17] Sashidhara, K. V.; Avula, S. R.; Mishra, V.; Palnati, G. R.; Singh, L. R.; Singh, N.; Chhonker, Y. S.; Swamy, P.; Bhatta, R. S.; Palit, G. Eur. J. Med. Chem. 2015, 89, 638.
[18] Yarishkin, O. V.; Ryu, H. W.; Park, J.; Yang, M. S.; Hong, S.; Park, K. H. Bioorg. Med. Chem. Lett. 2008, 18, 137.
[19] Wang, L.; Chen, G.; Lu, X.; Wang, S.; Hans, S.; Li, Y.; Ping, G.; Jiang, X.; Li, H.; Yang, J.; Wu, C. Eur. J. Med. Chem. 2015, 89, 88.
[20] Yamamoto, T.; Yoshimura, M.; Yamaguchi, F.; Kouchi, T.; Tsuji, R.; Saito, M.; Obata, A.; Kikuchi, M. Biosci. Biotechnol. Biochem. 2004, 68, 1706.
[21] Gao, H.; Zheng, X.; Qi, Y.; Wang, S.; Wan, C. P.; Rao, G. X.; Mao, Z. W. Chin. J. Org. Chem. 2018, 38, 648(in Chinese). (高慧, 郑喜, 祁燕, 王斯, 万春平, 饶高雄, 毛泽伟, 有机化学, 2018, 38, 648.)
[22] Thanusu, J.; Kanagarajan, V.; Gopalakrishnan, M. J. Enzyme Inhib. Med. Chem. 2010, 25, 347.
[23] Ramya, V.; Vembu, S.; Ariharasivakumar, G.; Gopalakrishnan, M. Pharma Chem. 2017, 9, 46.
[24] Arasakumar, T.; Mathusalini, S.; Ata, A.; Shankar, R.; Gopalan, S.; Lakshmi, K.; Sakthivel, P.; Mohan, P. S. Mol. Diversity 2017, 21, 37.
[25] Zhang, X.; Shi, G.; He, L.; Tao, K.; Hou, T. Pesticides 2012, 51, 301 (in Chinese). (张新刚, 史冠莹, 何利钦, 陶科, 侯太平, 农药, 2012, 51, 301.)
/
| 〈 |
|
〉 |