

Chinese Journal of Organic Chemistry >
Synthesis and Insecticidal Activity of Novel Piperidine Thiazole Compounds
Received date: 2018-09-05
Revised date: 2018-10-22
Online published: 2018-11-26
Supported by
Project supported by the Collaborative Innovation Center of Zhejiang Province Green Pesticide.
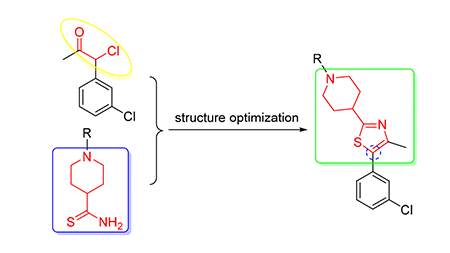
Twelve new piperdine thiazole compounds were designed and synthesized in search of new bioactive compounds. The preliminary bioassay showed that at the concentration of 500 μg/mL the lethal rates of the target compounds possessed certain insceticidal activities against armyworm, and at the concentration of 100 μg/mL the lethal rates of (4-(5-(3-chloro-phenyl) -4-methylthiazol-2-yl) piperidin-1-yl) (4-methylpiperazin-1-yl) methanone (1f) and (4-(5-(4-chlorophenyl) -4-methyl-thiazol-2-yl) piperidin-1-yl) nitro-1H-pyrazol-3-yl) methanone (1g) against armyworm were 80% and 100%, respectively. Further screening at concentrations of 20 μg/mL, the lethal rate of (4-(5-(4-chlorophenyl) -4-methylthiazol-2-yl) piperidin-1-yl) (4-nitro-1H-pyrazol-3-yl) methanone (1g) against armyworm was 50%.
Ding Chengrong , Pan Yayun , Yin Xu , Tan Chengxia , Zhang Guofu . Synthesis and Insecticidal Activity of Novel Piperidine Thiazole Compounds[J]. Chinese Journal of Organic Chemistry, 2019 , 39(3) : 836 -841 . DOI: 10.6023/cjoc201809009
[1] Huang, G.; Yang, J. -C.; Li, H.-C. Agrochemicals 2011, 50, 79(in Chinese.) (黄光, 杨吉春, 李慧超, 农药, 2011, 50, 79.)
[2] Yang, Z.-H.; Tian, H.; Zhang, L. World Pestic. 2017, 39, 43(in Chinese). (杨子辉, 田昊, 张莉, 世界农药, 2017, 39, 43.)
[3] Tan, Y.; Zhang, B.-H. J. Math. Med. 2012, 25, 89(in Chinese). (覃宇, 张宝徽, 数理医药学杂志, 2012, 25, 89.)
[4] Biller, S. A. US 5739135, 1998[Chem. Abstr. 1998, 128, 282780].
[5] Guedat, P. US 20070054939, 2007[Chem. Abstr. 2007, 142, 93809].
[6] Li, Y. CN 106588911, 2017[Chem. Abstr. 2017, 166, 515724].
[7] Fan, Z. J. CN 104650060, 2015[Chem. Abstr. 2015, 163, 65937].
[8] Gu, L.-L.; Bai, Y.-L. Mod. Agrochem. 2017, 16, 42(in Chinese.) (顾林玲, 柏亚罗, 现代农药, 2017, 16, 42.)
[9] He, X.-L. World Pestic. 2015, 37, 57(in Chinese). (何秀玲, 世界农药, 2015, 37, 57.)
[10] Cederbaum, F. WO 2014118142, 2014[Chem. Abstr. 2014, 161, 320959].
[11] Lamberth, C. WO 2014154530, 2014[Chem. Abstr. 2014, 161, 640429].
[12] Tomoki, T. US 20130296272, 2013[Chem. Abstr. 2012, 156, 337332].
[13] Gregory, V. WO 2009055514, 2009[Chem. Abstr. 2009, 150, 578441].
[14] Stefan, H. US 20160198713, 2016[Chem. Abstr. 2016, 162, 400022].
[15] Pierre, C. US 20110312999, 2011[Chem. Abstr. 2011, 155, 615369].
[16] Hanagan, M. A. WO 2009094407, 2009[Chem. Abstr. 2009, 151, 234956].
[17] Kamireddy, B. WO 2009094445, 2009[Chem. Abstr. 2009, 151, 173451].
[18] Pasteris, R. J. WO 2008013925, 2008[Chem. Abstr. 2008, 148, 185163].
[19] Yi, A.-Q.; Xue, S.-J.; Fang, Z.-K. Chin. J. Org. Chem. 2009, 29, 454(in Chinese). (尹安琴, 薛思佳, 方治坤, 有机化学, 2009, 29, 454.)
[20] Hong, Y.; Dai, H.; Ye, Y.-L. Chin. J. Org. Chem. 2017, 37, 3006(in Chinese). (洪宇, 戴红, 叶林玉, 有机化学, 2017, 37, 3006.)
[21] Gao, H.; Zheng, X.; Zhu, P. Chin. J. Org. Chem. 2018, 38, 684(in Chinese). (高慧, 郑喜, 朱萍, 有机化学, 2018, 38, 684.)
[22] Zhang, Z.-H.; Chen, Y.; Cai, B.-S. Chin. J. Org. Chem. 2017, 37, 2377(in Chinese). (张志华, 陈羽, 柴宝山, 有机化学, 2017, 37, 2377.)
[23] Cao, L.; Sun, J.-W.; Liu, Q. Chin. J. Org. Chem. 2017, 37, 3031(in Chinese). (曹蕾, 孙景伟, 刘强, 有机化学, 2017, 37, 3031.)
[24] Ma, H.-L.; Yan, X.-J.; Xiao, Y.-M. Chin. J. Org. Chem. 2016, 36, 158(in Chinese). (麻红利, 闫晓静, 肖玉梅, 有机化学, 2016, 36, 158.)
[25] Sun, N.; Wang, X.; Ding, Z.-B. Chin. J. Org. Chem. 2016, 36, 2489(in Chinese). (孙楠, 王欣, 丁志彬, 有机化学, 2016, 36, 2489.)
[26] Li, R.; Nakashima, T.; Galangau, O. Chem. Asian J. 2015, 10, 1725.
[27] Khillare, L. D.; Pratap, U. R.; Bhosle, M. R. Chem. Intermed. 2017, 12, 1.
[28] Tong, D.; Duan, H.; Wang, J. Chem. Res. Chin. Univ. 2014, 30, 4.
[29] Hantzsch, A.; Weber, J. H. Chem. Ber. 1887, 20, 3118.
[30] Parsons P. J.; Johnathan. B.; Waters A. J. Synth. Commun. 2007, 37, 985.
[31] Srivatsavati, Jagapathi, R.; Pothukuchi, S.; Rani, S.-S. WO 2012143933, 2012[Chem. Abstr. 2012, 167, 481902].
[32] Dai, H.; Ye, L. Y.; Zhuang, H. Y.; Dai, B. J.; Fang, Y.; Shi, Y. J. Molecules 2015, 20, 21870.
/
| 〈 |
|
〉 |