

Chinese Journal of Organic Chemistry >
Research Developments of the Construction of Chiral Center Based on Fluoroalkyl Radical Reactions
Received date: 2018-08-27
Revised date: 2018-11-21
Online published: 2018-11-30
Supported by
Project supported by the National Natural Science Foundation of China (No. 21332002).
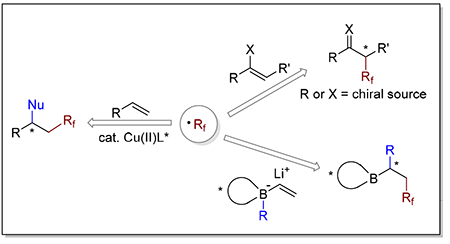
Fluorine-containing organic compounds have been widely applied in various fields, such as pharmacy, agrochemicals, materials science, etc. Trifluoromethyl, difluoromethyl and perfluoroalkyl groups represent the typical fluorine-containing functional groups. Hence, the development of highly efficient methods for introducing fluoroalkyl groups is of primary importance. In recent years, the reactions based on fluoroalkyl radicals have been developed into competent methods for introducing fluoroalkyl groups. On the other hand, the synthesis of the chiral molecules containing fluoroalkyl groups has attracted considerable attentions. However, attributed to the high reactivity of the fluoroalkyl radicals, the control of the reaction selectivity is difficult. Particularly, so far there have been only limited reports on the enantiocontrol of the reaction in this regard and the corresponding review of the field still lacks. This review summarizes the research on the asymmetric synthesis based on fluoroalkyl radical reactions developed over the past 20 years. The review introduces the selectivities, scope and mechanism of various reactions. It is divided into three different parts according to the type of the reaction: (1) asymmetric fluoroalkylation of enol/enamine intermediate through fluoroalkyl radical, (2) asymmetric difunctionalization of olefin through fluoroalkyl radical, (3) stereoselective 1,2-shift of vinylboronate complexes through fluoroalkyl radical.
Key words: fluoroalkylation; radicals; asymmetric synthesis; catalysis References
Huang Hang , Wang Xi , Wang Jianbo . Research Developments of the Construction of Chiral Center Based on Fluoroalkyl Radical Reactions[J]. Chinese Journal of Organic Chemistry, 2019 , 39(1) : 1 -14 . DOI: 10.6023/cjoc201808030
[1] (a) Furuya, T.; Kamlet, A. S.; Ritter, T. Nature 2011, 473, 470.
(b) Shimizu, M.; Hiyama, T. Angew. Chem., Int. Ed. 2005, 44, 214.
[2] Lehmann, F. Arch. Exp. Pathol. Pharmakol. 1928, 130, 250.
[3] Kirsch, P. Modern Fluoroorganic Chemistry, Synthesis, Reactivity, Applications, Wiley-VCH, Weinheim, 2004.
[4] (a) Ma, J.-A.; Cahard, D. Chem. Rev. 2004, 104, 6119.
(b) Ma, J.-A.; Cahard, D. Chem. Rev. 2008, 108, PR1.
(c) Nie, J.; Guo, H.-C.; Cahard, D.; Ma, J.-A. Chem. Rev. 2011, 111, 455.
[5] Dolbier, W. R. Chem. Rev. 1996, 96, 1557.
[6] (a) Studer, A. Angew. Chem., Int. Ed. 2012, 51, 8950.
(b) Wang, X.; Zhang, Y.; Wang, J. Sci. Sin. 2012, 42, 1417.
[7] Pan, X.; Xia, H.; Wu, J. Org. Chem. Front. 2016, 3, 1163.
[8] Wang, F.; Chen, P.; Liu, G. Acc. Chem. Res. 2018, 51, 2036.
[9] (a) Fessenden, R. W.; Schuler, R. H. J. Chem. Phys. 1965, 43, 2704.
(b) Krusic, P. J.; Bingham, R. C. J. Am. Chem. Soc. 1976, 98, 230.
[10] (a) Wang, S.-M.; Han, J.-B.; Zhang, C.-P.; Qin, H.-L.; Xiao, J.-C. Tetrahedron 2015, 71, 7949.
(b) Chatterjee, T.; Iqbal, N.; You, Y.; Cho, E. J. Acc. Chem. Res. 2016, 49, 2284.
(c) Wang, X.; Studer, A. Acc. Chem. Res. 2017, 50, 1712.
(d) Barata-Vallejo, S.; Cooke, M. V.; Postigo, A. ACS Catal. 2018, 8, 7287.
[11] (a) Iseki, K.; Nagai, T.; Kobayashi, Y. Tetrahedron Lett. 1993, 34, 2169.
(b) Iseki, K.; Nagai, T.; Kobayashi, Y. Tetrahedron:Asymmetry 1994, 5, 961.
[12] Itoh, Y.; Mikami, K. Tetrahedron 2006, 62, 7199.
[13] Nagib, D. A.; Scott, M. E.; MacMillan, D. W. C. J. Am. Chem. Soc. 2009, 131, 10875.
[14] Wozniak, L.; Murphy, J. J.; Melchiorre, P. J. Am. Chem. Soc. 2015, 137, 5678.
[15] Herrmann, A. T.; Smith, L. L.; Zakarian, A. J. Am. Chem. Soc. 2012, 134, 6976.
[16] Huo, H.; Huang, X.; Shen, X.; Harmsa, K.; Meggers, E. Synlett 2016, 27, 749.
[17] Liu, J.; Ding, W.; Zhou, Q.-Q.; Liu, D.; Lu, L.-Q.; Xiao, W.-J. Org. Lett. 2018, 20, 461.
[18] Yajima, T.; Nagano, H. Org. Lett. 2007, 9, 2513.
[19] Zhu, R.; Buchwald, S. L. Angew. Chem., Int. Ed. 2013, 52, 12655.
[20] Miller, Y.; Miao, L.; Hosseini, A. S.; Chemler, S. R. J. Am. Chem. Soc. 2012, 134, 12149.
[21] Wang, F.; Wang, D.; Wan, X.; Wu, L.; Chen, P.; Liu, G. J. Am. Chem. Soc. 2016, 138, 15547.
[22] Wu, L.; Wang, F.; Wan, X.; Wang, D.; Chen, P.; Liu, G. J. Am. Chem. Soc. 2017, 139, 2904.
[23] Fu, L.; Zhou, S.; Wan, X.; Chen, P.; Liu, G. J. Am. Chem. Soc. 2018, 140, 10965.
[24] Yu, P.; Lin, J.-S.; Li, L.; Zheng, S.-C.; Xiong, Y.-P.; Zhao, L.-J.; Tan, B.; Liu, X.-Y. Angew. Chem., Int. Ed. 2014, 53, 11890.
[25] Li, T.; Yu, P.; Du, Y.-M.; Lin, J.-S.; Zhi, Y.; Liu, X.-Y. J. Fluorine Chem. 2017, 203, 210.
[26] Lin, J.-S.; Dong, X.-Y.; Li, T.-T.; Jiang, N.-C.; Tan, B.; Liu, X.-Y. J. Am. Chem. Soc. 2016, 138, 9357.
[27] Lin, J.-S.; Wang, F.-L.; Dong, X.-Y.; He, W.-W.; Yuan, Y.; Chen, S.; Liu, X.-Y. Nat. Commun. 2017, 8, 14841.
[28] Cheng, Y.-F.; Dong, X.-Y.; Gu, Q.-S.; Yu, Z.-L.; Liu X.-Y. Angew. Chem., Int. Ed. 2017, 56, 8883.
[29] Li, X.-T.; Gu, Q.-S.; Dong, X.-Y.; Meng, X.; Liu, X.-Y. Angew. Chem., Int. Ed. 2018, 57, 7668.
[30] Kischkewitz, M.; Okamoto, K.; Mück-Lichtenfeld, C.; Studer, A. Science 2017, 355, 936.
[31] Gerleve, C.; Kischkewitz, M.; Studer, A. Angew. Chem., Int. Ed. 2018, 57, 2441.
/
| 〈 |
|
〉 |