

Chinese Journal of Organic Chemistry >
Synthesis of Alkyl Sulfate from α-Trifluoromethylbenzylbromide—An Extension of Sulfinatodehalogenation
Received date: 2018-10-18
Revised date: 2018-11-24
Online published: 2018-11-30
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21737004, 21672239, 21421002) and the Sanming Institute of Fluorochemical Industry (Nos. FCIT201704GR, FCIT201705GR, FCIT201701BR).
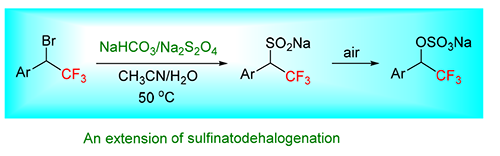
The sulfinatodehalogenation reaction is a common method of introducing a fluoroalkyl group. In this paper, the reaction of α-trifluoromethylbenzyl bromide under sulfinatodehalogenation conditions was investigated. It was found that the product was an sodium alkyl sulfate (ArCH(CF3)OSO3Na) instead of an sodium alkyl sulfinate (ArCH(CF3)SO2Na) which was normal produced. α-Trifluoromethylbenzyl bromide did not react with the olefin after its generation of a radical intermediate under sulfinatodehalogenation conditions even though an olefin was presented. Instead, the reaction directly gave an alkyl sulfinate, and then oxidized by air to provide a product as an alkyl sulfate.
Key words: Trifiluoromethyl; free radical; sulfinatodehalogenation; alkyl sulfate
Fu Xiaolin , Sun Yan , Zhao Zhigang , Guo Yong , Chen Qingyun , Nian Baoyi . Synthesis of Alkyl Sulfate from α-Trifluoromethylbenzylbromide—An Extension of Sulfinatodehalogenation[J]. Chinese Journal of Organic Chemistry, 2019 , 39(1) : 144 -150 . DOI: 10.6023/cjoc201810022
[1] (a) Huang, W.-Y. Organofluorine Chemistry in China, Shanghai Scientific and Technical Publishers, Shanghai, 1996 (in Chinese). (黄维垣, 中国有机氟化学研究, 上海科学技术出版社, 上海, 1996.)
(b) Zhang, C.-P.; Guo, Y.; Xiao, J.-C.; Gu, Y.-C. Chem. Soc. Rev. 2012, 41, 4536.
(c) Huang, B.-N.; Huang, W.-Y.; Hu, C.-M. Acta Chim. Sinica 1981, 39, 481(in Chinese). (黄炳南, 黄维垣, 胡昌明, 化学学报, 1981, 39, 481.)
(d) Huang, W.-Y.; Huang, B.-N.; Hu, C.-M. J. Fluorine Chem. 1983, 23, 193(in Chinese).
[2] (a) Qing, F.-L.; Qiu, X.-L. Organofluorine Chemistry, Science Press, Beijing, 2007(in Chinese). (卿凤翎, 邱小龙, 有机氟化学, 科学出版社, 北京, 2007.)
(b) Prakash, K. Modern Fluoroorganic Chemistry. Synthesis, Reactivity, Applications, 2nd ed., Wiley-VCH, Weinheim, 2013.
(c) Uneyama, K. Organofluorine Chemistry, Blackwell, Oxford, UK, 2006.
(d) Valentine, G. N.; Vasiliy, M. M.; Aleksey, V. S. Chem. Rev. 2015, 115, 973.
(e) Zhang, C.-P.; Chen, Q.-Y.; Guo, Y.; Xiao, J.-C.; Gu, Y.-C. Coord. Chem. Rev. 2014, 261, 28.
(f) Guo, Y.; Huang, M.-W.; Fu, X.-L.; Liu, C.; Chen, Q.-Y.; Zhao, Z.-G. Chin. Chem. Lett. 2017, 28, 719.
(g) Li, G.-M.; Sun, D.-Q. Chin. J. Org. Chem. 2016, 36, 1715(in Chinese). (李恭铭, 孙德群, 有机化学, 2016, 36, 1715.)
(h) He, J.-Q.; Lou, S.-J.; Xu, D.-Q. Acta Chim. Sinica 2016, 36, 1218(in Chinese). (何将旗, 娄绍杰, 许丹倩, 化学学报, 2016, 36, 1218.)
(i) Xu, Y.-Y.; Qian, A.-R.; Cao, X.-F.; Ling, C.-Y.; Cao, Y.-B.; Wang, R.-L.; Li, Y.-S.; Yang, Y.-S. Chin. Chem. Lett. 2016, 27, 703.
(j) Fu, L.-N.; Leng, B.; Li, Y.-S.; Gao, X.-K. Chin. Chem. Lett. 2016, 27, 1319.
[3] (a) Han, E.; Guo, Y.; Chen, Q.-Y.; Chin. J. Org. Chem. 2017, 37, 1714(in Chinese). (韩恩健, 郭勇, 陈庆云, 有机化学, 2017, 37, 1714.)
(b) Yang, B.; Xu, X.-H.; Qing, F.-L. Chin. J. Chem. 2016, 34, 465.
(c) Zhang, Z.; Yu, W.; Zhou, Q.; Li, T.; Zhang, Y.; Wang, J. Chin. J. Chem. 2016, 34, 473.
(d) Zhang, B.; Zhang, X.-G. Chin. J. Chem. 2016, 34, 477.
(e) Geng, Q.; Xiao, X.; He, G.-R.; Yao, S.-M.; Liang, G.-X. Chin. Chem. Lett. 2016, 27, 1009.
(f) Zhang, B.-H.; Kong, J.-J.; Huang, Y.; Lou, Y.-G.; Li, X.-F.; He, C.-Y. Chin. Chem. Lett. 2017, 28, 1751.
(g) Liu, Y.-L.; Yin, X.-P.; Zhou, J. Chin. J. Chem. 2018, 36, 321.
[4] (a) Miyake, Y.; Ota, S.-I.; Shibata, M.; Nakajima, K.; Nishibayashi, Y. Org. Biomol. Chem. 2014, 12, 5594.
(b) Egami, H.; Ide, T.; Kawato, Y.; Hamashima, Y. Chem. Commun. 2015, 51, 16675.
(c) Zhu, L.; Liu, S.; Douglas, J. T.; Altman, R. A. Chem. Eur. J. 2013, 19, 12800.
(d) Kuninobu, Y.; Nagase, M.; Kanai, M. Angew. Chem., Int. Ed. 2015, 54, 10263.
(e) Wang, X.; Song, S.; Jiao, N. Chin. J. Chem. 2018, 36, 213~216.
[5] (a) Vukovic, V. D.; Richmond, E.; Wolf, E.; Moran, J. Angew. Chem., Int. Ed. 2017, 56, 3085.
(b) Prakash, G. K. S.; Paknia, F.; Thomas M.; Mathew, T.; Mlostoń, G.; Olah, G. A. Org. Lett. 2011, 13, 4128.
[6] (a) Liang, Y.; Fu, G. C. J. Am. Chem. Soc. 2015, 137, 9523.
(b) Liang, Y.; Fu, G. C. Angew. Chem., Int. Ed. 2015, 54, 9047.
(c) Ryu, D.; Primer, D. N.; Tellis, J. C.; Molander, G. A. Chem. Eur. J. 2016, 22, 120.
(d) Li, X.; Feng, Z.; Jiang, Z.-X.; Zhang, X. Org. Lett. 2015, 17, 5570.
[7] (a) Gao, B.; Zhao, Y.; Hu, J. Angew. Chem., Int. Ed. 2015, 54, 638.
(b) Tang, H.-J.; Zhang, Y.-F.; Jiang, Y.-W.; Feng, C. Org. Lett. 2018, 20, 5190.
(c) Tian, P.; Wang, C.-Q.; Cai, S.-H.; Song, S.; Ye, L.; Feng, C.; Loh, T.-P. J. Am. Chem. Soc. 2016, 138, 15869.
(d) Tang, H.-J.; Lin, L.-Z.; Feng, C.; Loh T.-P. Angew. Chem., Int. Ed. 2017, 56, 9872.
[8] (a) Long, Z.-Y., Chen, Q.-Y. Tetrahedron Lett. 1998, 39, 8487.
(b) Long, Z.-Y.; Chen, Q.-Y. J. Org. Chem. 1999, 64, 4775.
[9] (a) Li, L.; Huang, M.; Liu, C.; Xiao, J.-C.; Chen, Q.-Y.; Guo, Y.; Zhao, Z.-G. Org. Lett. 2015, 17, 4714.
(b) Huang M.; Li, L.; Zhao, Z.-G.; Chen, Q.-Y.; Guo, Y. Synth. Catal. 2015, 47, 3891.
(c) Kreis, L. M.; Krautwald, S.; Pfeiffer, N.; Martin, R. E.; Carreira, E. M. Org. Lett. 2013, 15, 1634.
(d) Rong, J.; Ni, C.; Wang, Y.; Kuang, C.; Gu, Y.; Hu, J. Acta Chim. Sinica 2017, 75, 105.
[10] Huan, F., Chen, Q.-Y., Guo, Y. J. Org. Chem. 2016, 81, 7051.
[11] (a) Kudova, E.; Chodounska, H.; Slavikova, B.; Budesinsky, M.; Nekardova, M.; Vyklicky, V.; Krausova, B.; Svehla, P.; Vyklicky, L. J. Med. Chem. 2015, 58, 5950.
(b) Burlingham, B. T.; Pratt L. M.; Davidson, E. R.; Shiner, V. J.; Jr.; Fong, J.; Widlanski, T. S. J. Am. Chem. Soc. 2003, 125, 13036.
[12] Kamiyama, H.; Kubo, Y.; Sato, H.; Yamamoto, N.; Fukuda, T.; Ishibashi, F.; Iwao, M. Bioorg. Med. Chem. 2011, 19, 7541.
[13] (a) Stastnaa, E.; Chodounskaa, H.; Pouzara, V.; Kaprasa, V.; Borovskaa, J.; Caisc, O.; Jr, L. V. Steroids 2009, 74, 256.
(b) Nishimura, Y.; Shudo, H.; Seto, H.; Hoshino, Y.; Miura, Y. Bioorg. Med. Chem. Lett. 2013, 23, 6390.
[14] (a) Nie, L.; S. Yao; Dong, B.; Li, X.-l.; Song, H. J. Mol. Liq. 2017, 240, 152.
(b) Desoky, A. Y.; Hendel, J.; Ingram L.; Taylor, S. D. Tetrahedron 2011, 67, 1281.
(c) Beichel, W.; Panzer, J. M. U.; Hätty, J. Ye, X.; Himmel, D.; Krossing, I. Angew. Chem., Int. Ed. 2014, 53, 6637.
[15] Dolbier, W. R.; Jr. Guide to Fluorine NMR for Organic Chemists, John Wiley & Sons, Inc., New Jersey, 2009, p. 143.
[16] (a) Brunelle, J. A.; Letendre, L. J.; Weltin, E. E.; Brown, J. H.; Bushweller, C. H. J. Phys. Chem. 1992, 96, 9225.
(b) Tordeux, M.; Wakselman, C.; Jarjayes, O.; Béguin, C. G. Magn. Reson. Chem. 2001, 39, 301.
[17] Wang, R.; Jiang, L.; Yi, W. J. Org. Chem. 2018, 83, 7789.
[18] Dneprovskii, A. S.; Eliseenkov, E. V.; Mil'tsov, S. A. J. Org. Chem. 1982, 2, 365.
[19] Yusuke, Y.; Shoji, H.; Hisanori, S. Tetrahedron 2010, 2, 473.
[20] Okano, T; Fumoto, M; Kusukawa, T; Fujita, M. J. Fluorine Chem. 2002, 9, 91.
[21] Andreea, A. O.; Daweon, R.; Ioana, A.; Gary A, M. Angew. Chem., Int. Ed. 2013, 51, 13901.
[22] Lo, W. C.; Hunter, J. E.; Watson, G. B.; Patny, A.; Iyer, P. S.; Boruwa, J. US 9211280, 2015.
[23] Sun, F.; Zhan, Y. CN 108341814, 2018.
/
| 〈 |
|
〉 |