

Chinese Journal of Organic Chemistry >
Trifluoromethylated-Imidazolines as Efficient Organocatalyst for Asymmetric Aldol Reaction of Hydroxyacetone with Aldehydes
Received date: 2018-09-21
Revised date: 2018-12-05
Online published: 2018-12-07
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21572128, 21672139) and the Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Science.
Aldol reaction of hydroxyacetone is an all-purpose route to construct the 1,2-diol building blocks for the synthesis of multifarious natural products and biological active molecules. In this work, a new series of trifluoromethylated-imidazoline organocatalysts have been designed and synthesized. It is found that the trifluoromethylated chiral organocatalyst (2R,4S)-4-benzyl-1,2-dimethyl-2-(trifluoromethyl) imidazolidine (1a) has proved to be very efficient for the direct asymmetric aldol reaction of α-hydroxyketones with aldehydes to build the syn-1,2-diol building blocks. Among the synthesized syn-aldol products, a good yield (up to 96%) and high stereoselectivity (up to dr=15:1, 99% ee) could be obtained. The F—H bonding derived from trifluoromethyl group was proposed to play an important role in the stabilization of the transition state.
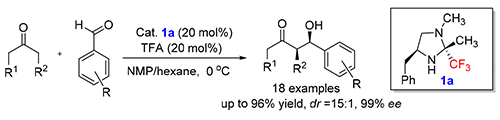
Key words: asymmetric aldol reaction; organocatalysis; imidazoline; trifluoromethyl; synthesis
Xie Xiaojuan , Zhang Zhong , Zhao Huaxin , Wan Wen , Hao Jian . Trifluoromethylated-Imidazolines as Efficient Organocatalyst for Asymmetric Aldol Reaction of Hydroxyacetone with Aldehydes[J]. Chinese Journal of Organic Chemistry, 2019 , 39(1) : 117 -121 . DOI: 10.6023/cjoc201809026
[1] For reviews, see:(a) Palomo, C.; Oiarbide, M.; Garcia, J. M. Chem. Soc. Rev. 2004, 33, 65.
(b) Mahrwald, R. Modern Aldol Reactions, Wiley-VCH, Weinheim, Germany, 2004, Vols. 1~2.
(c) Dalko, P. I.; Moisan, L. Angew. Chem., Int. Ed. 2004, 43, 5138.
(d) Houk, K. N.; List, B. Acc. Chem. Res. 2004, 37, 487.
(e) List, B. Chem. Rev. 2007, 107, 5413.
[2] (a) List, B.; Lerner, R. A. Barbas. C. F. J. Am. Chem. Soc. 2000, 122, 2395.
(b) Notz, W.; List, B. J. Am. Chem. Soc. 2000, 122, 7386.
[3] For reviews, see:(a) Torii, H.; Nakadai, M.; Ishihara, K.; Saito, S.; Yamamoto, H. Angew. Chem., Int. Ed. 2004, 43, 1983.
(b) Northrup, A. B.; Mangion, I. K.; Hettche, F.; MacMillan, D. W. C. Angew. Chem., Int. Ed. 2004, 43, 2152.
(c) Northrup, A. B.; MacMillan, D. W. C. Science 2004, 305, 1752.
(d) Cordova, A.; Zou, W.; Ibrahem, I.; Reyes, E.; Engqvist, M.; Liao, W. W. Chem. Commun. 2005, 3586.
(e) Kano, T.; Takai, J.; Tokuda, O.; Maruoka, K. Angew. Chem., Int. Ed. 2005, 44, 3055.
(f) Enders, D.; Grondal, C. Angew. Chem., Int. Ed. 2005, 44, 1210.
(g) Suri, J. T.; Ramachary, D. B.; Barbas. C. F. Org. Lett. 2005, 7, 1383.
(h) Mase, N.; Nakai, Y.; Ohara, N.; Yoda, H.; Takabe, K.; Tanaka, F.; Barbas. C. F. J. Am. Chem. Soc. 2006, 128, 734.
(i) Mukherjee, S.; Yang, J. W.; Hoffman, S.; List, B. Chem. Rev. 2007, 107, 5471.
(j) Li, J.-Y.; Luo, S.-Z.; Cheng, J.-P. J. Org. Chem. 2009, 74, 1747.
(k) Vellalath, S.; Romo, D. Angew. Chem., Int. Ed. 2016, 55, 13934.
(l) Frias, M.; Cieslik, W.; Fraile, A.; Rosado-Abon, A.; Garido-Castro, A. F.; Yuste, F.; Aleman, J. Chem.-Eur. J. 2018, 24, 10906.
[4] For examples of syn-aldol reactions of ketones, see:(a) Ramasastry, S. V.; Zhang, H.; Tanaka, F.; Barbas. C. F. J. Am. Chem. Soc. 2007, 129, 288.
(b) Luo, S.; Xu, H.; Li, J.; Zhang, L.; Cheng, J.-P. J. Am. Chem. Soc. 2007, 129, 3074.
(c) Ramasastry, S. S. V.; Albertshofer, K.; Utsumi, N.; Tanaka, F.; Barbas. C. F. Angew. Chem., Int. Ed. 2007, 46, 5572.
(d) Xu, X.-Y.; Wang, Y.-Z.; Gong, L.-Z. Org. Lett. 2007, 9, 4247.
(e) Utsumi, N.; Imai, M.; Tanaka, F.; Ramasastry, S. S. V.; Barbas. C. F. Org. Lett. 2007, 9, 3445.
(f) Ramasastry, S. S. V.; Albertshofer, K.; Utsumi, N.; Barbas. C. F. Org. Lett. 2008, 10, 1621.
(g) Zhu, M.-K.; Xu, X.-Y.; Gong, L.-Z. Adv. Synth. Catal. 2008, 350, 1390.
(h) Luo, S.; Xu, H.; Zhang, L.; Li, J.; Cheng, J.-P. Org. Lett. 2008, 10, 653.
[5] (a) Purser, S.; Moore, P. R.; Swallow S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320.
(b) Begue, J. P.; Bonnet-Delpon, D. Bioorganic and Medicinal Chemistry of Fluorine, Wiley, Hoboken, 2008.
(c) Ojima, I. Fluorine in Medicinal Chemistry and Chemical Biology, John Wiley & Sons, Chichester, 2009.
(d) Wan, W.; Ma, G.; Li, J.; Chen, Y.; Hu, Q.; Li, M.; Jiang, H.; Deng, H.; Hao, J. Chem. Commun. 2016, 52, 1598.
(e) Wang, J.; Sanchez-Rosello, M.; Acena J.; Sorochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Liu, H. Chem. Rev. 2014, 114, 2432.
(f) Lee, K. A.; Silverio, D. L.; Torker, S.; Robbins, D. W.; Haeffner, F.; Mei, F. W.; Hoveyda, A. H. Nat. Chem. 2016, 8, 768.
(g) Rong, J.; Ni, C.; Wang, Y.; Kuang, C.; Gu, Y.; Hu, J. Acta Chim. Sinica 2017, 75, 105(in Chinese). (荣健, 倪传法, 王云泽, 匡翠文, 顾玉诚, 胡金波, 化学学报, 2017, 75, 105.)
(h) Xu, X.; Chen, H.; He, J.; Xu, H. Chin. J. Chem. 2017, 35, 1665.
(i) Hui, R.; Zhang, S.; Tan, Z.; Wu, X.; Feng, B. Chin. J. Org. Chem. 2017, 37, 3060(in Chinese). (惠人杰, 张士伟, 谭政, 吴小培, 冯柏年, 有机化学, 2017, 37, 3060.)
[6] (a) O'Hagan, D.; Bilton, C.; Howard, J. A. K.; Knight, L.; Tozer, D. J. J. Chem. Soc., Perkin Trans. 2 2000, 605.
(b) Briggs, C. R. S.; O'Hagan, D.; Howard, J. A. K.; Yufit, D. S. J. Fluorine Chem. 2003, 119, 9.
(c) Gooseman, N. E. J.; O'Hagan, D.; Slawin, A. M. Z.; Teale, A. M.; Tozer, D. J.; Young, R. J. Chem. Commun. 2006, 3190.
(d) Gooseman, N. E. J.; O'Hagan, D.; Peach, M. G.; Slawin, A. M. Z.; Tozer, D. J.; Young, R. J. Angew. Chem., Int. Ed. 2007, 46, 5904.
(e) MacMillan, D. W. C. Nature 2008, 455.
(f) Cahard, D.; Bizet, V. Chem. Soc. Rev. 2014, 43, 135.
[7] (a) Sparr, C.; Schweizer, W. B.; Senn, H. M.; Gilmou, R. Angew. Chem., Int. Ed. 2009, 48, 3065.
(b) Diocco, D. A.; Oberg, K. M.; Dalton, D. M.; Rovis. T. J. Am. Chem. Soc. 2009, 131, 10872.
[8] For reviews, see:(a) List, B.; Shabat, D.; Barbas. C. F.; Lerner, R. A. Chem.-Eur. J. 1999, 4, 881.
(b) Yoshikawa, N.; Kumagai, N.; Matsunaga, S.; Moll, G.; Ohshima, T.; Suzuki, T.; Shibasaki, M. J. Am. Chem. Soc. 2001, 123, 2466.
(c) Trost, B. M.; Ito, H.; Silcoff, E. R. J. Am. Chem. Soc. 2001, 123, 3367.
(d) Kumagai, N.; Matsunaga, S.; Kinoshita, T.; Harada, S.; Okada, S.; Sakamoto, S.; Yamaguchi, K.; Shibasaki, M. J. Am. Chem. Soc. 2003, 125, 2169.
[9] Ahrendt, K. A.; Borths, C. J.; MacMillan. D. W. C. J. Am. Chem. Soc. 2000, 122, 4243.
[10] (a) Sarka, D.; Harman, K.; Ghosh, S.; Headley, D. Tetrahedron:Asymmetry 2011, 22, 1051.
(b) Czarnecki, P.; Plutecka, A.; Gawronski, J.; Kacprzak, K. Green Chem. 2011, 13, 1280.
(c) Paradowska, J.; Pasternak, M.; Gut, B.; Gryzlo, B.; Mlynarski, J. J. Org. Chem. 2012, 77, 173.
/
| 〈 |
|
〉 |