

Chinese Journal of Organic Chemistry >
Synthesis and Biological of Novel Myricetin Derivatives Containing 1,3,4-Oxadiazoles
Received date: 2018-09-28
Revised date: 2018-11-02
Online published: 2018-12-17
Supported by
Project supported by the National Key Research and Development Program of China (No.2017YFD0200506),and the National Natural Science Foundation of China (No.21867003).
A series of novel myricetin derivatives containing 1,3,4-oxadiazole moiety were designed and synthesized.Bioassays indicated that some compounds showed potential antibacterial and antiviral activities. Among them, compounds 4a,4b, 4f and 4j demonstrated appreciable inhibitory effect against Xanthomonas axonopodis pv.citri (Xac), with half-maximal effective concentration (EC50) values of 18.5, 40.7, 26.9 and 32.4 μg/mL, which were significantly better than commercial agent bismerthiazol (68.8 μg/mL), compounds 4f and 4j also demonstrated appreciable inhibitory effect against Xanthomonas oryzae pv. Oryzae (Xoo) with EC50 values of 45.9 and 35.7 μg/mL, which were better than commercial agent bismerthiazol (69.3 μg/mL). In addition, compounds 4n demonstrated significant curative activity against TMV with EC50 value of 272.8 μg/mL, which was better than commercial agent ningnamycin (428.8 μg/mL), compounds 4f showed protecting activity against tobacco mosaic virus (TMV) with EC50 value of 235.6 μg/mL, which was better than commercial agent ningnamycin (447.9 μg/mL). Microscale thermophoresis (MST) indicated that compound 4j could bind with south rice black drawf virus P9-1.
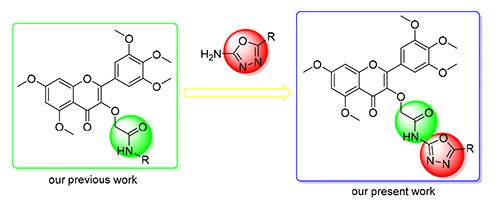
Key words: myricetin; oxadiazole; biological activity; protein
Zhang Cheng , Jiang Shichun , Chen Ying , Guo Tao , Xia Rongjiao , Tang Xu , Chen Lijuan , He Ming , Xue Wei . Synthesis and Biological of Novel Myricetin Derivatives Containing 1,3,4-Oxadiazoles[J]. Chinese Journal of Organic Chemistry, 2019 , 39(4) : 1160 -1168 . DOI: 10.6023/cjoc201809040
[1] Bos, L. Trends Microbiol. 2000, 8, 82.
[2] Li, X. Y.; Liu, J.; Yang, X.; Ding, Y.; Wu, J.; Hu, D. Y.; Song, B. A. Bioorg. Med. Chem. Lett. 2015, 23, 3629.
[3] Zhou, G. H.; Wen, J. J.; Cai, D. J.; Li, P.; Xu, D. L.; Zhang, S. G. Chin. Sci. Bull. 2008, 53, 3677.
[4] Huang, N.; Angeles, E. R.; Domingo, J.; Magpanty, G.; Singh, S.; Zhan, G.; Kumarvadivel, N.; Bennett, J.; Khush, G. S. Theor. Appl. Genet. 1997, 95, 313.
[5] Guo, R.-X.; Li, L.-G.; Wang, Y.-F.; Huo, C.-H.; Fu, Y.; Wang, L.; Shi, W.-Q. Chin. Tradit. Herbal Drugs 2015, 46, 2019(in Chinese).(郭瑞霞, 李力更, 王于方, 霍长虹, 付炎, 王磊, 史文清, 中草药, 2015, 46, 2019.)
[6] Liu, C.-L.; Li, Z.-M. Pesticides 2003, 42, 1(in Chinese).(刘长令, 李正名, 农药, 2003, 42, 1.)
[7] Mei, Q.-G.; Yuan, W.-C.; Wang, C. Chin. J. Org. Chem. 2015, 35, 70(in Chinese).(梅青刚, 袁伟成, 王淳, 有机化学, 2015, 35, 70.)
[8] Yu, M. S.; Lee, J.; Lee, J. M.; Kim, Y.; Chin, Y. W.; Jee, J. G.; Keum, Y. S.; Jeong, Y. J. Bioorg. Med. Chem. Let. 2012, 22, 4049.
[9] Nguyen, T. H. V.; Trinh, A. V.; Nguyen, X. N.; Kiem, P. V.; Minh, C. V.; Long, P. Q.; Anh, L.T.; Cuong, N. M.; Song, J. H.; Ko, H. J.; Kim, N, Park, S. J.; Kim, S. H. Nat. Prod. Commun. 2014, 9, 643.
[10] Zhong, X. M.; Wang, X. B.; Chen, L. J.; Ruan, X. H.; Li, Q.; Zhang, J. P.; Chen, Z.; Xue, W. Chem. Cent. J. 2017, 11, 106.
[11] Chen, C. C; Huang, C. Y. Protein J. 2011, 30, 59.
[12] Rashed, K.; Ciric, A.; Glamoclija, J.; Sokovic, M. Ind. Crop. Prod. 2014, 59, 210.
[13] Xiao, W.; Ruan, X.-H.; Li, Q.; Zhang, J.-P.; Zhong, X.-M.; Xie, Y.; Wang, X.-B.; Huang, M.-G.; Xue, W. Chem. J. Chin. Univ. 2017, 38, 35(in Chinese).(肖维, 阮祥辉, 李琴, 张菊平, 钟新敏, 谢艳, 王晓斌, 黄民国, 薛伟, 高等学校化学学报, 2017, 38, 35.)
[14] Chobot, V.; Hadacek, F. Redox Rep. 2011, 16, 242.
[15] Zhao, L.; Xu, S. P.; Li, Z. Y.; Zhang, L.; Zhang, Z. S.; Pan, R. L. Sci. Technol. Food Ind. 2012, 33, 56.
[16] Xue, W.; Song, B. A.; Zhao, H. J. Eur. J. Med. Chem. 2015, 97, 155.
[17] Tae, K. H.; Inae, J.; Mi, E. K.; Bae, S. K.; Lee, J. K. Biomed. Pharmacother. 2017, 91, 378.
[18] Huang, M.-G.; Ruan, X.-H.; Zhang, J.-P.; Li, Q.; Wang, Y.-H.; Chen, L.-J.; Zhang, C.; Li, P. Chin. J. Org. Chem. 2017, 37, 2145(in Chinese).(黄民国, 阮祥辉, 张菊平, 李琴, 王一会, 陈丽娟, 张橙, 李普, 有机化学, 2017, 37, 2145.)
[19] Ningaiah, S.; Bhadraiah, U. K.; Doddaramappa, S. D.; Keshavamurthy, K. Bioorg. Med. Chem. Lett. 2014, 24, 245.
[20] Li, P.; Shi, L.; Gao, M. L.; Yang, X.; Xue, W.; Jin, L. H.; Hu, D. Y.; Song, B. A. Bioorg. Med. Chem. Lett. 2015, 25, 481.
[21] Gan, X. H.; Hu, D. Y.; Chen, Z.; Wang, Y. J.; Song, B. A. Bioorg. Med. Chem. Lett. 2017, 27, 4298.
[22] Wu, W. N; Chen, Q.; Tai, A. Q.; Jiang, G. Q.; Ouyang, G. P. Bioorg. Med. Chem. Lett. 2015, 25, 2243.
[23] Ragab, F. A. F.; Abou-Seri, S. M.; Abdel-Aziz, S. A.; Alfayomy, A. M; Aboelmagd, M. Eur. J. Med. Chem. 2017, 138, 140
[24] Zhang, S.; Luo, Y.; He, L. Q.; Liu, Z. J.; Jiang, A. Q.; Yang, Y. H.; Zhu, H. L. Bioorg. Med. Chem. Lett. 2013, 21, 3723..
[25] Aziz-Ur-Rehman; Siddiqui, S. Z.; Abbasi, M. A.; Abbas, N.; Khan, K. M.; Shahid, M.; Mahmood, Y. Int. J. Pharm. Pharm. Sci. 2012, 4, 676.
[26] Rajak, H.; Kharya, M. D.; Mishra, P. Arch. Pharm. Chem. Life Sci. 2008, 341, 247.
[27] Niu, P. F.; Kang, J. F.; Tian, X. H.; Song, L. N.; Liu, H. X.;Wu, J.; Yu, W. Q.; Chang, J. B. J. Org. Chem. 2015, 80, 1018.
/
| 〈 |
|
〉 |