

Chinese Journal of Organic Chemistry >
Three-Component Synthesis of Chromeno[4,3-d]pyrazolo[3,4-b]-pyridine Derivatives and Their Fluorescence Properties
Received date: 2018-09-29
Revised date: 2018-12-10
Online published: 2018-12-21
Supported by
Project supported by the Major Basic Research Project of the Natural Science Foundation of the Jiangsu Higher Education Institutions (Nos.15KJA150006,17KJA150006).
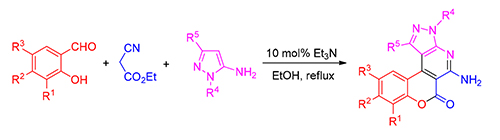
A series of 5-aminochromeno[4,3-d]pyrazolo[3,4-b]pyridin-6(3H)-one derivatives 4a~4w were synthesized by three-component reactions of salicylaldehydes, ethyl cyanoacetate and 5-aminopyrazoles catalyzed by triethylamine (TEA). The properties of the synthesized compounds were examined by fluorescence spectroscopy and the results indicated that some compounds were possessed high fluorescence quantum yields.
Zhang Mengye , Wang Ning , Xu Wentao , Huang Zhibin , Shi Daqing . Three-Component Synthesis of Chromeno[4,3-d]pyrazolo[3,4-b]-pyridine Derivatives and Their Fluorescence Properties[J]. Chinese Journal of Organic Chemistry, 2019 , 39(4) : 1085 -1094 . DOI: 10.6023/cjoc201809042
[1] (a) Shi, Y.; Zhou, C. H. Bioorg. Med. Chem. Lett. 2011, 21, 956.
(b) Laxmi, S. V.; Kuarm, B. S.; Rajitha, B. Med. Chem. Res. 2013, 22, 768.
(c) Varun, G.; Bhila, C. V.; Patel, N. H.; Patel, D. I. B. Med. Chem. Res. 2013, 22, 4338.
[2] Hemshekhar, M.; Sunitha, K.; Thushara, R. M.; Santhosh, S.; Sundaram, M. S.; Kemparaju, K.; Girish, K. S. Biochimie 2013, 95, 1326.
[3] (a) Li, W.; Sun, Y. N.; Yan, X. T.; Yang, S. Y.; Kim, E. J.; Kang, H. K.; Kim, Y. H. J. Agric. Food Chem. 2013, 61, 10730.
(b) Francisco, C. S.; Rodrigues, L. R.; Cerqueira, N. M.; Oliveira-Campos, A. M.; Rodrigues, L. M. Eur. J. Med. Chem. 2012, 47, 370.
(c) Li, B.; Liu, Q. X.; Zhou, Y. Q.; Jia, Z. D.; Zhu, M. Y.; Xu, Y.; Song, M. P. Chin. J. Org. Chem. 2017, 37, 2008(in Chinese).(李标, 刘秋霞, 周元清, 贾赵栋, 朱曼毓, 徐琰, 宋毛平, 有机化学, 2017, 37, 2008.)
(d) Huang, X. W.; Liu, J. L. Chin. J. Org. Chem. 2018, 38, 1233(in Chinese).(黄新炜, 刘建利, 有机化学, 2018, 38, 1233.)
[4] Daly, A. K. Arch. Toxicol. 2013, 87, 407.
[5] (a) Kirkiacharian, S.; Thuy, D. T.; Sicsic, S. Farmaco 2002, 57, 703.
(b) Kashman, Y. J. Med. Chem. 1992, 35, 2735.
[6] (a) Lin, Q. H.; Bao, C. Y.; Cheng, S. Y.; Yang, Y. L.; Ji, W.; Zhu, L. Y. J. Am. Chem. Soc. 2012, 134, 5052.
(b) Hirano, T.; Kubo, H.; Shiraishi, T.; Hiromoto, K.; Fujiwara, T.; Kagechika, H. Tetrahedron Lett. 2012, 53, 5916.
(c) Sanap, K. K.; Samant, S. D. Tetrahedron Lett. 2012, 53, 5407.
[7] (a) Zhao, Y. R.; Zheng, Q.; Dakin, K.; Xu, K.; Martinez, M. L.; Li, W. H. J. Am. Chem. Soc. 2004, 126, 4653.
(b) Lee, K. S.; Kim, H. J.; Kim, G. H.; Shin, I.; Hong, J. I. Org. Lett. 2008, 10, 49.
(c) Huang, Q.; Qu, W. J.; Chen, J.; Lin, Q.; Yao, H.; Zhang, Y. M.; Wei, T. B. Chin. J. Org. Chem. 2018, 38, 629(in Chinese).(黄青, 曲文娟, 陈洁, 林奇, 姚虹, 张有明, 魏太保, 有机化学, 2018, 38, 629.)
[8] Chen, J. H.; Liu, W. M.; Ma, J. J.; Xu, H. T.; Wu, J. S.; Tang, X. L.; Fan, Z. Y.; Wang, P. F. J. Org. Chem. 2012, 77, 3475.
[9] (a) Liu, X. C.; Lin, W.; Wang, H. Y.; Huang, Z. B.; Shi, D. Q. J. Heterocycl. Chem. 2014, 51, 1036.
(b) Wang, J. X.; Lin, W.; Liu, H. T.; Hu, M. H.; Feng, X.; Ren, J. F.; Huang, Z. B.; Shi, D. Q. Chin. J. Org. Chem. 2015, 35, 927(in Chinese).(王菊仙, 林伟, 刘洪涛, 胡明华, 冯贤, 任金凤, 黄志斌, 史达清, 有机化学, 2015, 35, 927.)
[10] (a) You, X. W.; Yu, H.; Wang, M. G.; Wu, J.; Shang, Z. C. Lett. Org. Chem. 2012, 9, 19.
(b) Jin, P. P.; Liu, X. C.; Liu, D. Q.; Huang, Z. B.; Shi, D. Q. J. Heterocycl. Chem. 2015, 52, 1625.
(c) Lin, W.; Cai, Q.; Zheng, C. Z.; Zheng, Y. X.; Shi, D. Q. Chin. J. Org. Chem. 2017, 37, 2392(in Chinese).(林伟, 蔡琦, 郑纯智, 郑永祥, 史达清, 有机化学, 2017, 37, 2392.)
[11] (a) Posner, G. H. Chem. Rev. 1986, 86, 831.
(b) Armstrong, R. W.; Combs, A. P.; Tempest, P. A.; Brown, S. D.; Keating, T. A. Acc. Chem. Res. 1996, 29, 123.
(c) Wan, J. P.; Gan, L.; Liu, Y. Org. Biomol. Chem. 2017, 15, 9031.
(d) Wan, J. P.; Liu, Y. RSC Adv. 2012, 2, 9763.
[12] (a) Sarkar, S.; Khan, A. T. Chem. Commun. 2015, 51, 12673.
(b) Guo, W. S.; Xin, X.; Zhao, K. L.; Wen, L. R.; Li, M. RSC Adv. 2015, 5, 70429.
(c) Jiang, B.; Fan, W.; Sun, M. Y.; Ye, Q.; Wang, S. L.; Tu, S. J.; Li, G. J. Org. Chem. 2014, 79, 5258.
(d) Shi, G. H.; He, X. W.; Shang, Y. J.; Xiang, L. W.; Yang, C.; Han, G.; Du, B. Chin. J. Chem. 2016, 34, 901.
(e) Xiao, M.; Yan, C. G. Chin. J. Chem. 2017, 35, 1422.
(f) Gao, G.; Wang, P.; Liu, P.; Zhag, W. H.; Mo, L. P.; Zhang, Z. H. Chin. J. Org. Chem. 2018, 38, 846(in Chinese).(高歌, 王萍, 刘鹏, 张卫红, 默丽萍, 张占辉, 有机化学, 2018, 38, 846.)
(g) Wan, J. P.; Lin, Y.; Huang, Q.; Liu, Y. J. Org. Chem. 2014, 79, 7232.
(h) Wan, J. P.; Gao, S.; Liu, Y. J. Org. Chem. 2015, 80, 9028.
(i) Wan, J. P.; Lin, Y.; Jing, Y.; Xu, M.; Liu, Y. Tetrahedron 2014, 70, 7874.
[13] (a) Feng, X.; Wang, J. J.; Xun, Z.; Zhang, J. J.; Huang, Z. B.; Shi, D. Q. Chem. Commun. 2015, 51, 1528.
(b) Feng, X.; Wang, J. J.; Xun, Z.; Huang, Z. B.; Shi, D. Q. J. Org. Chem. 2015, 80, 1025.
(c) Wang, J. J.; Feng, X.; Xun, Z.; Shi, D. Q.; Huang, Z. B. J. Org. Chem. 2015, 80, 8435.
(d) Xun, Z.; Feng, X.; Wang, J. J.; Shi, D. Q.; Huang, Z. B. Chin. J. Chem. 2016, 34, 696.
/
| 〈 |
|
〉 |