

Chinese Journal of Organic Chemistry >
Design, Synthesis, and Antifungal Activity of Novel Thiochromanone Derivatives Containing 1,3,4-Oxadiazole Skeleton
Received date: 2018-08-16
Revised date: 2018-11-13
Online published: 2018-12-28
Supported by
Project supported by the Natural Science Foundation of Hebei Province (No.B2018201269),the National Natural Science Foundation of China (No.21675039),the China Postdoctoral Science Foundation (No.2016M591401),the Young Talent of Hebei Province,and the Hebei University Science Fund for Distinguished Young Scholars (No.2015JQ06).
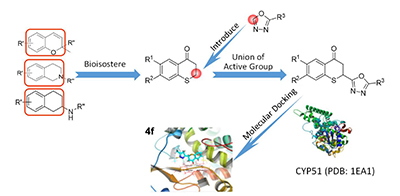
Following the principle of union of active group, the thiochromanone was combined with 1,3,4-oxadiazole, and 12 compounds were designed and synthesized. The target compounds were confirmed by 1H NMR and HRMS. The preliminary antifungal activity assay showed that most of the target compounds exhibited significant inhibition activity against four animal pathogenic fungi and four plant pathogenic fungi. Among them, the minimum inhibitory concentration (MIC) value of compound 4f against Canidia albicans reached 4 μg·mL-1, and the MIC value of 4d against Aspergillusnigervan tiegh reached 8 μg·mL-1, which were higher than the positive controls. And the molecular docking studies have found that 4f has strong binding ability to CYP51 of Canidia albicans, which may be a potential CYP51 inhibitor.
Key words: thiochromanone; 1,3,4-oxadiazole; antifungal; molecular docking
Sun Xiaoyang , Feng Jiajia , Li Shengbin , Feng Siran , Liu Zhenming , Song Yali , Qiao Xiaoqiang . Design, Synthesis, and Antifungal Activity of Novel Thiochromanone Derivatives Containing 1,3,4-Oxadiazole Skeleton[J]. Chinese Journal of Organic Chemistry, 2019 , 39(4) : 1037 -1043 . DOI: 10.6023/cjoc201808016
[1] Song, J. L.; Jones, L. M.; Chavarria, G. E.; Pinney, K. G. Bioorg. Med. Chem. Lett. 2013, 23, 2801.
[2] Wei, P.; Liu, J. J.; Ma, J. J.; Yang, G. L. Mod. Appl. Sci. 2010, 12, 136.
[3] Liu, Y.; Luo, W.; Sun, L. Drug. Discovery Ther. 2008, 2, 216.
[4] Fang, B. L.; Ma, Z. Y.; Yang, G. L. Int. J. Chem. 2010, 2, 143.
[5] Ma, Z. Y.; Zhang, X. H.; Wang, S. K.; He, Y.; Yang, G. L. Chin. J. Org. Chem. 2011, 29, 757(in Chinese).(马正月, 张兴华, 王士奎, 何洋, 杨更亮, 有机化学, 2011, 29, 757.)
[6] Qi, P.; Le, Y. H.; Guo, C.; Fang, L. Chin. J. Med. Chem. 2003, 13, 205(in Chinese).(齐平, 靳颖华, 郭春等, 方林, 中国药物化学杂志, 2003, 13, 205.)
[7] Song, Y. L.; Yang, T.; Dong, Y. F; Wu. F; Yang, G. L. Chem. Lett. 2014, 43, 134.
[8] Wang, G.; Ma, Z. Y.; Tian, W.; Yang, G. L. Int. J. Chem. 2010, 2, 19.
[9] Gibson, M. S. Tetrahedron 1962, 18, 1377.
[10] Rehman, A. U.; Ahtzaz, S.; Abbasi, M. A. J. Chil. Chem. Soc. 2017, 62, 3370.
[11] Szulczyk, D.; Tomaszewski, P.; Jozwiak, M.; Koziol, A. E.; Lis, T.; Collu, D. Molecules 2017, 22, 409.
[12] Kerzare, D.; Chikhale, R.; Bansode, R. J. Braz. Chem. Soc. 2016, 27, 1998.
[13] Islam, M. U.; Albratty, M. J. J. Chem. Biol. Phys. Sci., Sect. A:Chem. Sci. 2016, 6, 624.
[14] Khan, M. S. Y.; Chawla, G.; Mueed, M. A. Indian J. Chem., Sect. B 2004, 43, 1302.
[15] Song, Y. L.; Liu, X. M.; Yang, N.; Yang, G. L. Asian J. Chem. 2013, 25, 1849.
[16] Zhu, J.; Sheng, C. Q.; Zhang, W. N. Acta Pharm. Sin. 2005, 40, 775(in Chinese).(朱杰, 盛春泉, 张万年, 药学学报, 2005, 40, 775.)
[17] Song, Y. L.; Yang, T.; Dong, Y., F.; Wu, F.; Yang, G. L. Chem. Lett. 2014, 43, 134.
[18] Miura, T.; Klaus, W.; Ross, A.; Senn, H. Eur. J. Biochem. 2001, 268, 4833.
[19] Chen, G.; Deng, Q.; Tang, Y.; Chen, S. J. Chin. J. Chem. 2010, 18, 74(in Chinese).(陈刚, 邓强, 汤颖, 陈世军, 合成化学, 2010, 18, 74.)
[20] Lepesheva, G. I.; Waterman, M. R. Biochim. Biophys. Acta 2007, 1770, 467.
[21] Lepesheva, G. I.; Waterman, M. R. Curr. Top. Med. Chem. 2011, 11, 2060.
[22] Georgopapadakou, N. H.; Walsh, T. J. Antimicrob. Agents Chemother. 1996, 40, 279.
[23] Ji, H. T.; Zhang, W. N.; Zhou, Y. J.; Zhu, J.; Lu, J. G.; Zhu, J. J. Med. Chem. 2000, 43, 2493.
[24] Zhu, J.; Lu, J. G.; Zhou, Y. J.; Li, Y. W.; Cheng, J.; Zheng, C. H. Bioorg. Med. Chem. Lett. 2006, 16, 5285.
[25] Yao, B.; Ji, H. T.; Cao, Y. B.; Zhou, Y. J.; Zhu, J.; Lu, J. G.; Li, Y. W.; Chen, J.; Zheng, C. H.; Jiang, Y. Y.; Liang, R. M.; Tang, H. J. Med. Chem. 2007, 50, 5293
[26] Liang, G. C.; Zhou, G.; Zhong, Y. F.; Han, X. Y.; Song, Y. L. Chin. J. Synth. Chem. 2015, 23, 1100(in Chinese).(梁国超, 周冠, 钟一凡, 韩晓燕, 宋亚丽, 合成化学, 2015, 23, 1100.)
[27] Taha, M.; Ismail, N. H.; Imran, S.; Wadood, A.; Rahim, F.; Khan, K. M. Nasir, A. Bioorg. Chem. 2016, 66, 117.
[28] Wani. M, Y.; Ahmad. A.; Shiekh, R. A.; Al-Ghamdi, K.; Sobral, A. Bioorg. Med. Chem. 2015, 23, 4172.
[29] Karegoudar, P.; Karthikeyan, M. S.; Prasad, D. J.; Mahalinga, M.; Holla, B. S. Kumari, N. S. Eur. J. Med. Chem. 2008, 43, 261.
[30] Zhu, X. F.; Shi, D. Q. Phosphorus Sulfur 2008, 183, 2020.
/
| 〈 |
|
〉 |