

Chinese Journal of Organic Chemistry >
Recent Developments in Protein Engineering and Catalytic Oxidations of Baeyer-Villiger Monooxygenase
Received date: 2018-10-19
Revised date: 2018-12-13
Online published: 2018-12-28
Supported by
Project supported by the National Natural Science Foundation of China (Nos.21472169,21574113).
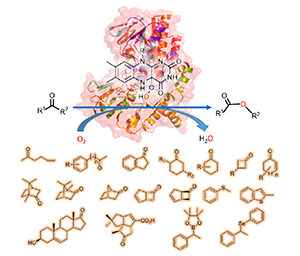
Baeyer-Villiger monooxygenase (BVMO) is an important biocatalyst for Baeyer-Villiger oxidation of various organic ketone/aldehyde compounds, and sulfur, selenium, or boron-containing heteroatoms compounds. As an indispensable tool, BVMO-catalyzed oxidation displays some advantages, such as high selectivity, mild reaction conditions and high efficiency, leading to wide applications into the synthesis of chiral compounds. In recent years, bioinformatics analysis and genome mining have been used to find more novel BVMOs from microorganisms. Besides natural substrates, these BVMOs can accept various organic compounds showing wide substrate scope. Meanwhile, protein engineering has been widely used to improve the catalytic performance of BVMOs, such as the expanded substrate scope, high thermostability and activity, high stereo-, regio-and chemo-selectivities. Based on the Baeyer-Villiger oxidation reaction with different substrate structures, the recent advancements in the research on the catalytic oxidation of wild type and protein-engineered BVMOs in the past five years are summarized.
Zheng He , Zhou Yuke , Lin Xianfu , Wu Qi . Recent Developments in Protein Engineering and Catalytic Oxidations of Baeyer-Villiger Monooxygenase[J]. Chinese Journal of Organic Chemistry, 2019 , 39(4) : 903 -915 . DOI: 10.6023/cjoc201810023
[1] Balke, K.; Kadow, M.; Mallin, H.; Sass, S.; Bornscheuer, U. T. Org. Biomol. Chem. 2012, 10, 6249.
[2] Leisch, H.; Morley, K.; Lau, P. C. Chem. Rev. (Washington, DC, U. S.) 2011, 111, 4165.
[3] (a) Dong, J.; Fernandez-Fueyo, E.; Hollmann, F.; Paul, C. E.; Pesic, M.; Schmidt, S.; Wang, Y.; Younes, S.; Zhang, W. Angew. Chem., Int. Ed. 2018, 57, 9238.
(b) Balke, K.; Beier, A.; Bornscheuer, U. T. Biotechnol. Adv. 2018, 36, 247.
(c) Liang, Y.; Wei, J.; Qiu, X.; Jiao, N. Chem. Rev. 2018, 118, 4912.
[4] Ceccoli, R. D.; Bianchi, D. A.; Fink, M. J.; Mihovilovic, M. D.; Rial, D. V. AMB Express 2017, 7, 87.
[5] Beneventi, E.; Niero, M.; Motterle, R.; Fraaije, M.; Bergantino, E. J. Mol. Catal. B:Enzym. 2013, 98, 145.
[6] Fiorentini, F.; Romero, E.; Fraaije, M. W.; Faber, K.; Hall, M.; Mattevi, A. ACS Chem Biol. 2017, 12, 2379.
[7] Fink, M. J.; Mihovilovic, M. D. Chem. Commun. (Cambridge, U. K.) 2015, 51, 2874.
[8] van Beek, H. L.; Romero, E.; Fraaije, M. W. ACS Chem Biol. 2017, 12, 291.
[9] Pereira, J. P. C.; van der Wielen, L. A. M.; Straathof, A. J. J. Bioresour. Technol. 2018, 256, 187.
[10] Carvalho, A. T. P.; Dourado, D.; Skvortsov, T.; de Abreu, M.; Ferguson, L. J.; Quinn, D. J.; Moody, T. S.; Huang, M. Phys. Chem. Chem. Phys. 2018, 20, 2558.
[11] Song, J. W.; Jeon, E. Y.; Song, D. H.; Jang, H. Y.; Bornscheuer, U. T.; Oh, D. K.; Park, J. B. Angew. Chem., Int. Ed. 2013, 52, 2534.
[12] Jeon, E.-Y.; Seo, J.-H.; Kang, W.-R.; Kim, M.-J.; Lee, J.-H.; Oh, D.-K.; Park, J.-B. ACS Catal. 2016, 6, 7547.
[13] Seo, E. J.; Yeon, Y. J.; Seo, J. H.; Lee, J. H.; Bongol, J. P.; Oh, Y.; Park, J. M.; Lim, S. M.; Lee, C. G.; Park, J. B. Bioresour. Technol. 2018, 251, 288.
[14] (a) Rehdorf, J.; Zimmer, C. L.; Bornscheuer, U. T. Appl. Environ. Microbiol. 2009, 75, 3106.
(b) Geitner, K.; Rehdorf, J.; Snajdrova, R.; Bornscheuer, U. T. Appl. Microbiol. Biotechnol. 2010, 88, 1087.
[15] Riebel, A.; Dudek, H. M.; de Gonzalo, G.; Stepniak, P.; Rychlewski, L.; Fraaije, M. W. Appl. Microbiol. Biotechnol. 2012, 95, 1479.
[16] Ferroni, F. M.; Smit, M. S.; Opperman, D. J. J. Mol. Catal. B:Enzym. 2014, 107, 47.
[17] (a) Fraaije, M. W.; Wu, J.; Heuts, D. P.; van Hellemond, E. W.; Spelberg, J. H.; Janssen, D. B. Appl. Microbiol. Biotechnol. 2005, 66, 393.
(b) de Gonzalo, G.; Mihovilovic, M. D.; Fraaije, M. W. ChemBioChem 2010, 11, 2208.
[18] Pazmino, D. E. T.; Snajdrova, R.; Rial, D. V.; Mihovilovic, M. D.; Fraaije, M. W. Adv. Synth. Catal. 2007, 349, 1361.
[19] Dudek, H. M.; de Gonzalo, G.; Pazmino, D. E.; Stepniak, P.; Wyrwicz, L. S.; Rychlewski, L.; Fraaije, M. W. Appl. Environ. Microbiol. 2011, 77, 5730.
[20] Dudek, H. M.; Fink, M. J.; Shivange, A. V.; Dennig, A.; Mihovilovic, M. D.; Schwaneberg, U.; Fraaije, M. W. Appl. Microbiol. Biotechnol. 2014, 98, 4009.
[21] Franceschini, S.; van Beek, H. L.; Pennetta, A.; Martinoli, C.; Fraaije, M. W.; Mattevi, A. J. Biol. Chem. 2012, 287, 22626.
[22] Bisagni, S.; Summers, B.; Kara, S.; Hatti-Kaul, R.; Grogan, G.; Mamo, G.; Hollmann, F. Top. Catal. 2013, 57, 366.
[23] Messiha, H. L.; Ahmed, S. T.; Karuppiah, V.; Suardiaz, R.; Ascue Avalos, G. A.; Fey, N.; Yeates, S.; Toogood, H. S.; Mulholland, A. J.; Scrutton, N. S. Biochemistry 2018, 57, 1997.
[24] Alexander, A. K.; Biedermann, D.; Fink, M. J.; Mihovilovic, M. D.; Mattes, T. E. J. Mol. Catal. B:Enzym. 2012, 78, 105.
[25] Fink, M. J.; Fischer, T. C.; Rudroff, F.; Dudek, H.; Fraaije, M. W.; Mihovilovic, M. D. J. Mol. Catal. B:Enzym. 2011, 73, 9.
[26] Rudroff, F.; Fink, M. J.; Pydi, R.; Bornscheuer, U. T.; Mihovilovic, M. D. Monatsh. Chem. 2017, 148, 157.
[27] Balke, K.; Schmidt, S.; Genz, M.; Bornscheuer, U. T. ACS Chem Biol. 2016, 11, 38.
[28] Zhang, Z. G.; Parra, L. P.; Reetz, M. T. Chem.-Eur. J. 2012, 18, 10160.
[29] Rodríguez-Mata, M.; Lavandera, I.; Gotor-Fernández, V.; Gotor, V.; García-Cerrada, S.; Mendiola, J.; de Frutos, Ó.; Collado, I. Tetrahedron 2016, 72, 7268.
[30] Reetz, M. T.; Brunner, B.; Schneider, T.; Schulz, F.; Clouthier, C. M.; Kayser, M. M. Angew. Chem., Int. Ed. 2004, 43, 4075.
[31] Clouthier, C. M.; Kayser, M. M.; Reetz, M. T. J. Org. Chem. 2006, 71, 8431.
[32] Polyak, I.; Reetz, M. T.; Thiel, W. J. Phys. Chem. B 2013, 117, 4993.
[33] Zhang, Z.-G.; Roiban, G.-D.; Acevedo, J. P.; Polyak, I.; Reetz, M. T. Adv. Synth. Catal. 2013, 355, 99.
[34] Parra, L. P.; Agudo, R.; Reetz, M. T. ChemBioChem 2013, 14, 2301.
[35] Wu, S.; Acevedo, J. P.; Reetz, M. T. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 2775.
[36] Yachnin, B. J.; McEvoy, M. B.; MacCuish, R. J.; Morley, K. L.; Lau, P. C.; Berghuis, A. M. ACS Chem Biol. 2014, 9, 2843.
[37] Iwaki, H.; Grosse, S.; Bergeron, H.; Leisch, H.; Morley, K.; Hasegawa, Y.; Lau, P. C. Appl. Environ. Microbiol. 2013, 79, 3282.
[38] Schmidt, S.; Genz, M.; Balke, K.; Bornscheuer, U. T. J. Biotechnol. 2015, 214, 199.
[39] Mallin, H.; Wulf, H.; Bornscheuer, U. T. Enzyme Microb. Technol. 2013, 53, 283.
[40] Staudt, S.; Bornscheuer, U. T.; Menyes, U.; Hummel, W.; Groger, H. Enzyme Microb. Technol. 2013, 53, 288.
[41] Oberleitner, N.; Peters, C.; Rudroff, F.; Bornscheuer, U. T.; Mihovilovic, M. D. J. Biotechnol. 2014, 192, 393.
[42] Schmidt, S.; Scherkus, C.; Muschiol, J.; Menyes, U.; Winkler, T.; Hummel, W.; Groger, H.; Liese, A.; Herz, H. G.; Bornscheuer, U. T. Angew. Chem., Int. Ed. 2015, 54, 2784.
[43] Milker, S.; Fink, M. J.; Oberleitner, N.; Ressmann, A. K.; Bornscheuer, U. T.; Mihovilovic, M. D.; Rudroff, F. ChemCatChem 2017, 9, 3420.
[44] Kohl, A.; Srinivasamurthy, V.; Bottcher, D.; Kabisch, J.; Bornscheuer, U. T. Enzyme Microb. Technol. 2018, 108, 53.
[45] Reignier, T.; de Berardinis, V.; Petit, J. L.; Mariage, A.; Hamze, K.; Duquesne, K.; Alphand, V. Chem. Commun. (Cambridge, U. K.) 2014, 50, 7793.
[46] Morrill, C.; Jensen, C.; Just-Baringo, X.; Grogan, G.; Turner, N. J.; Procter, D. J. Angew. Chem., Int. Ed. 2018, 57, 3692.
[47] Kadow, M.; Loschinski, K.; Sass, S.; Schmidt, M.; Bornscheuer, U. T. Appl. Microbiol. Biotechnol. 2012, 96, 419.
[48] Fink, M. J.; Rial, D. V.; Kapitanova, P.; Lengar, A.; Rehdorf, J.; Cheng, Q.; Rudroff, F.; Mihovilovic, M. D. Adv. Synth. Catal. 2012, 354, 3491.
[49] Leipold, F.; Wardenga, R.; Bornscheuer, U. T. Appl. Microbiol. Biotechnol. 2012, 94, 705.
[50] Kadow, M.; Balke, K.; Willetts, A.; Bornscheuer, U. T.; Backvall, J. E. Appl. Microbiol. Biotechnol. 2014, 98, 3975.
[51] Furst, M. J.; Savino, S.; Dudek, H. M.; Gomez Castellanos, J. R.; Gutierrez de Souza, C.; Rovida, S.; Fraaije, M. W.; Mattevi, A. J. Am. Chem. Soc. 2017, 139, 627.
[52] Balke, K.; Baumgen, M.; Bornscheuer, U. T. ChemBioChem 2017, 18, 1627.
[53] Butinar, L.; Mohorcic, M.; Deyris, V.; Duquesne, K.; Iacazio, G.; Claeys-Bruno, M.; Friedrich, J.; Alphand, V. Phytochemistry 2015, 117, 144.
[54] Romero, E.; Castellanos, J. R.; Mattevi, A.; Fraaije, M. W. Angew. Chem., Int. Ed. 2016, 55, 15852.
[55] van Beek, H. L.; de Gonzalo, G.; Fraaije, M. W. Chem. Commun. (Cambridge, U. K.) 2012, 48, 3288.
[56] Mascotti, M. L.; Palazzolo, M. A.; Bisogno, F. R.; Kurina-Sanz, M. Steroids 2016, 109, 44.
[57] Bosserman, M. A.; Downey, T.; Noinaj, N.; Buchanan, S. K.; Rohr, J. ACS Chem Biol. 2013, 8, 2466.
[58] Chen, K.; Wu, S.; Zhu, L.; Zhang, C.; Xiang, W.; Deng, Z.; Ikeda, H.; Cane, D. E.; Zhu, D. Biochemistry 2016, 55, 6696.
[59] Minerdi, D.; Zgrablic, I.; Castrignano, S.; Catucci, G.; Medana, C.; Terlizzi, M. E.; Gribaudo, G.; Gilardi, G.; Sadeghi, S. J. Antimicrob. Agents Chemother. 2016, 60, 64.
[60] Qiao, K.; Chooi, Y. H.; Tang, Y. Metab. Eng. 2011, 13, 723.
[61] Hu, Y.; Dietrich, D.; Xu, W.; Patel, A.; Thuss, J. A.; Wang, J.; Yin, W. B.; Qiao, K.; Houk, K. N.; Vederas, J. C.; Tang, Y. Nat. Chem. Biol. 2014, 10, 552.
[62] de Gonzalo, G.; Torres Pazmiño, D. E.; Ottolina, G.; Fraaije, M. W.; Carrea, G. Tetrahedron:Asymmetry 2006, 17, 130.
[63] (a) Branchaud, B. P.; Walsh, C. T. J. Am. Chem. Soc. 1985, 107, 2153.
(b) Walsh, C. T.; Chen, Y. C. J. Angew. Chem., Int. Ed. Engl. 1988, 27, 333.
[64] Gonzalo, G. D.; Pazmiño, D. E. T.; Ottolina, G.; Fraaije, M. W.; Carrea, G. Tetrahedron:Asymmetry 2005, 16, 3077.
[65] Ceccoli, R. D.; Bianchi, D. A.; Rial, D. V. Front. Microbiol. 2014, 5, 25.
[66] Brondani, P. B.; de Gonzalo, G.; Fraaije, M. W.; Andrade, L. H. Adv. Synth. Catal. 2011, 353, 2169.
[67] Das, B. C.; Thapa, P.; Karki, R.; Schinke, C.; Das, S.; Kambhampati, S.; Banerjee, S. K.; Van Veldhuizen, P.; Verma, A.; Weiss, L. M.; Evans, T. Future Med. Chem. 2013, 5, 653.
[68] Alphand, V.; Wohlgemuth, R. Curr. Org. Chem. 2010, 14, 1928.
[69] Bordewick, S.; Beier, A.; Balke, K.; Bornscheuer, U. T. Enzyme Microb. Technol. 2018, 109, 31.
[70] de Gonzalo, G.; Franconetti, A. Enzyme Microb. Technol. 2018, 113, 24.
[71] Mascotti, M. L.; Juri Ayub, M.; Dudek, H.; Sanz, M. K.; Fraaije, M. W. AMB Express 2013, 3, 33.
[72] Zhai, X. H.; Ma, Y. H.; Lai, D. Y.; Zhou, S.; Chen, Z. M. J. Ind. Microbiol. Biotechnol. 2013, 40, 797.
[73] Zhang, Z. G.; Lonsdale, R.; Sanchis, J.; Reetz, M. T. J. Am. Chem. Soc. 2014, 136, 17262.
[74] Dudek, H. M.; Popken, P.; van Bloois, E.; Duetz, W. A.; Fraaije, M. W. J. Biomol. Screening. 2013, 18, 678.
[75] Nikodinovic-Runic, J.; Coulombel, L.; Francuski, D.; Sharma, N. D.; Boyd, D. R.; Ferrall, R. M.; O'Connor, K. E. Appl. Microbiol. Biotechnol. 2013, 97, 4849.
[76] Zhang, Y.; Liu, F.; Xu, N.; Wu, Y. Q.; Zheng, Y. C.; Zhao, Q.; Lin, G.; Yu, H. L.; Xu, J. H. Appl. Environ. Microbiol. 2018, 84.
[77] Andrade, L. H.; Pedrozo, E. C.; Leite, H. G.; Brondani, P. B. J. Mol. Catal. B:Enzym. 2011, 73, 63.
[78] Brondani, P. B.; Guilmoto, N. M. A. F.; Dudek, H. M.; Fraaije, M. W.; Andrade, L. H. Tetrahedron 2012, 68, 10431.
/
| 〈 |
|
〉 |