

Chinese Journal of Organic Chemistry >
Unexpected Rearrangement Reaction and Synthesis of Benzoxazoles
Received date: 2018-09-28
Revised date: 2018-11-14
Online published: 2019-01-10
Supported by
Project supported by the National Natural Science Foundation of China (Nos.21776060,21276064),and the Natural Science Foundation of Hebei Province (No.B2016205165).
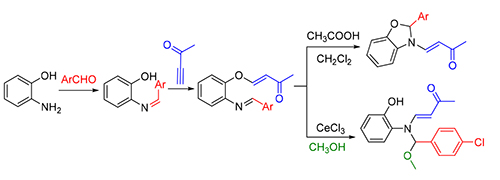
Novel series of rearrangement reactions were herein reported that enable access to a variety of unique 2-aryl-3-(3'-oxobutenyl)-benzoxazole compounds 6a~6f from 2-aminophenol, aromatic aldehyde and 3-butyn-2-one as materials by nucleophilic conjugate addition, dehydration and rearrangement reactions and intramolecular cyclization in the presence of a catalytic amount of CH3COOH in CH2Cl2 at ambient temperature. On the basis of products and intermediate products, a series of possible mechanism was presented and theoretically verified by density functional theory (DFT) method at B3LYP/6-31G (d,p) level from both molecular energy and atomic charge in Gaussian 03 package. The results show that the theory and experiment consistently explain the rationality of the reaction mechanism. The mechanism of rearrangement provides a basis for further study of this type of reaction. The advantage of this method is that a novel structure of benzoxazole derivative was synthesized successfully via a series of rearrangement reactions. Therefore, this method can be used as an attractive strategy for practical synthesis of nitrogen heterocyclic compounds.
Wang Kaixuan , Wang Lanzhi . Unexpected Rearrangement Reaction and Synthesis of Benzoxazoles[J]. Chinese Journal of Organic Chemistry, 2019 , 39(4) : 1147 -1152 . DOI: 10.6023/cjoc201809038
[1] Zhou, W. J.; Zhang, L.; Xiao, W.; Chen, H. J.; Wu, W. N.; Ouyang, G. P. J. Heterocycl. Chem. 2017, 54, 1423.
[2] Praveen, C.; Nandakumar, A.; Dheenkumar, P.; Muralidharan, D.; P. Perumal, P. T. J. Chem. Sci. 2012, 124, 609.
[3] Temiz-Arpaci, O.; Arisoy, M.; Sac, D.; Doganc, F.; Tasci, M.; Senol, F. S.; Orhan, I. E. Z. Naturforsch., C 2016, 71, 409.
[4] Reen, G. K,; Kumar, A.; Sharma, P. Med. Chem. Res. 2017, 26, 3336.
[5] Yildiz-Oren, I.; Yalcin, I.; Aki-Sener, E.; Ucarturk, N. Eur. J. Med. Chem. 2004, 9, 291.
[6] Tasci, M.; Temiz-Arpaci, O.; Kaynak-Onurdag, F.; Okten, S. Indian J. Chem., Sect. B:Org. Chem. Incl. Med. Chem. 2018, 57, 385.
[7] Tseng, C.-H.; Lin, C.-K.; Chen, Y.-L.; Tseng, C.-K.; Lee, J.-Y. Lee, J.-C. Eur. J. Med. Chem. 2018, 143, 970.
[8] Henderson, J. A.; Bilimoria, D.; Bubenik, M.; Cadilhac, C.; Cottrell, K. M.; Denis, F.; Dietrich, E.; Ewing, N.; Falardeau, G.; Giroux, S.; L'Heureux, L.; Liu, B.; Mani, N.; Morris, M.; Nicolas, O.; Pereira, O. Z.; Poisson, C.; Reddy, T. J.; Selliah, S.; Shawgo, R. S.; Vaillancourt, L.; Wang, J.; Xu, J.; Chauret, N.; Berlioz-Seux, F.; Chan, L. C.; Das, S. K.; Grillot, A.-L.; Bennani, Y. L.; Maxwell, J. P. Bioorg. Med. Chem. Lett. 2015, 25, 948.
[9] Zilifdar, F.; Foto, E.; Ertan-Bolelli, T.; Aki-Yalcin, E.; Yalcin, I.; Diril, N. Arch. Pharm. 2018, 351.
[10] Khajondetchairit, P.; Phuangsawai, O.; Suphakun, P.; Rattanabunyong, S.; Choowongkomon, K.; Gleeson, M. P. Chem. Biol. Drug Des. 2017, 90, 987.
[11] Goekhan-Kelekci, N.; Koeksal, M.; Uenuevar, S.; Aktay, G.; Erdogan, H. J. Enzyme Inhib. Med. Chem. 2009, 24, 29.
[12] Eren, G.; Unlu, S.; Nunez, M. T.; Labeaga, L.; Ledo, F.; Entrena, A.; Banoglu, E.; Costantino, G.; Sahin, M. F. Bioorg. Med. Chem. 2010, 18, 6367.
[13] Jayanna, N. D.; Vagdevi, H. M.; Dharshan, J. C.; Raghavendra, R. Telkar, S. B. Med. Chem. Res. 2013, 22, 5814.
[14] Wei, P.-F.; Qi, M.-Z.; Wang, Z.-P.; Ding, S.-Y.; Yu, W.; Liu, Q.; Wang, L.-K.; Wang, H.-Z.; An, W.-K.; Wang, W. J. Am. Chem. Soc. 2018, 140, 4623.
[15] Yeh, V. S. C. Tetrahedron 2004, 60, 11995.
[16] Leaver, I. H.; Milligan, B. Dyes Pigm. 1984, 5, 109.
[17] Chen, T.-R. J. Organomet. Chem. 2008, 693, 3117.
[18] Dunwell, D. W.; Evans, D. Hicks, T. A. J. Med. Chem. 1975, 18, 1158.
[19] Grossi, G.; Di Braccio, M.; Roma, G.; Ballabeni, V.; Tognolini, M.; Calcina, F.; Barocelli, E. Eur. J. Med. Chem. 2002, 37, 933
[20] Smith, R. H., Jr.; Jorgensen, W. L.; Tirado-Rives, J. Lamb, M. L.; Janssen, P. A.; Michejda, C. J.; Kroeger Smith, M. B. J. Med. Chem. 1998, 41, 5272
[21] Liu, D.; Chen, H. Y.; Zhang, J. Y.; Huang, J. Y.; Li, Y. M.; Peng, Q. M. Appl. Surf. Sci. 2018, 456, 59.
[22] Li, X.-Q. Li; Wang, L.-Z. Chin. Chem. Lett. 2014, 25, 327.
[23] Yin, L.-Y.; Wang, L.-Z. Tetrahedron Lett. 2016, 57, 5935.
[24] Qiu, Z.-L.; Wang, L.-Z.; Li, W.-H.; Li. Y. Acta Chim. Sinica 2011, 69, 1217(in Chinese).(邱召来, 王兰芝, 李文红, 李媛, 化学学报, 2011, 69, 1217.)
/
| 〈 |
|
〉 |