

Chinese Journal of Organic Chemistry >
Recent Progress in Transition Metal-Catalyzed Coupling Reactions of Organotitanium Reagents
Received date: 2018-10-29
Revised date: 2018-12-19
Online published: 2019-01-10
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21762018, 21772067), the Science and Technology Project Founded by the Education Department of Jiangxi Province (No. GJJ160668), the Program of Qingjiang Excellent Young Talents, Jiangxi University of Science and Technology, the Innovation and Entrepreneurship Training Program (No. XZG-16-08-12) and the Doctoral Scientific Research Foundation of Jiangxi University of Science and Technology.
Organotitanium proves to be one of ideal organometallic candidates because of its low price, non-toxicity, diversified types, excellent chemo-, regio- and stereo-selectivities. The reactivity of organotitanium reagent could be easily controlled by ligands of central titanium atom. Recently, the coupling reactions of organotitanium reagent have attracted extensive attention. This review summerized recent progress in transiton metal-catalyzed coupling reactons of organotitanium reagents concerning their types.
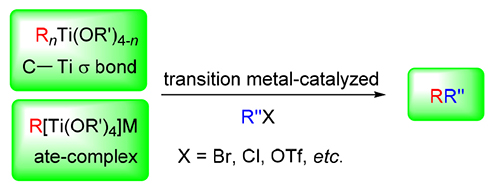
Key words: transition metal; catalysis; organotitanium reagent; coupling
Huang Hui , Li Juanhua , Liu Kunming , Liu Jinbiao . Recent Progress in Transition Metal-Catalyzed Coupling Reactions of Organotitanium Reagents[J]. Chinese Journal of Organic Chemistry, 2019 , 39(5) : 1293 -1303 . DOI: 10.6023/cjoc201810036
[1] For Grignard reagent:(a) Tamao, K. J. Organomet. Chem. 2002, 653, 23.
(b) Knochel, P.; Dohle, W.; Gommermann, N.; Kneisel, F. F.; Kopp, F.; Korn, T.; Sapountzis, I.; Vu, V. A. Angew. Chem., Int. Ed. 2003, 42, 4302.
(c) Ha, H.; Baron, O.; Wanger, A. J.; Knochel, P. Chem. Lett. 2006, 35, 2.
[2] For Lithium reagent:(a) Barluenga, J.; Feunandez, A.; Alvarez- Rodrigo, L.; Rodriguez, F.; Fananas, F. J. Synlett 2005, 2513.
(b) Son, E. C.; Tsuji, H.; Saeki, T.; Tamao, K. Bull. Chem. Soc. Jpn. 2006, 79, 492.
(c) He, P.; Dong, C. G.; Hu, Q. S. Tetrahedron Lett. 2008, 49, 1906.
[3] Knochel, P.; Singer, R. D. Chem. Rev. 1993. 93, 2117.
[4] Lipshutz, B. H. Acc. Chem. Res. 1997, 30, 277.
[5] (a) Miyaura, N.; Suzuki, A. Chem. Rev. 1995, 95, 2457.
(b) Suzuki, A. Pure Appl. Chem. 1994, 66, 213.
[6] Espinet, P.; Echavarren, A. M. Angew. Chem., Int. Ed. 2004, 43, 4704.
[7] Weidmann, B.; Seebach, D. Angew. Chem., Int. Ed. Engl. 1983, 12, 31.
[8] (a) Reetz, M. T.; Steinbach, R.; Westermann, J.; Peter, R. Angew. Chem., Int. Ed. 1980, 92, 1044.
(b) Olivero, A. G.; Weidmann, B.; Seebach, D. Helv. Chim. Acta 1981, 64, 2485.
(c) Widler, L.; Seebach, D. Helv. Chim. Acta 1982, 65, 1085.
(d) Widler, L.; Seebach, D. Helv. Chim. Acta 1982, 65, 1972.
[9] (a) Wu, S. Z.; Zhou, Y. K. Chin. J. Org. Chem. 1983, 3, 9(in Chinese). (吴绍祖, 周耀坤, 有机化学, 1983, 3, 9.)
(b) Chen, M. W. Chem. Reag. 1992, 14, 297(in Chinese). (陈美文, 化学试剂, 1992, 14, 297.)
(c) Hughes, D. L. Top. Organomet. Chem. 2004, 6, 37.
(d) Kulinkovich, O. G.; Meijere, A. D. Chem. Rev. 2000, 100, 2789;
(e) Hayashi, T. Bull. Chem. Soc. Jpn. 2004, 77, 13.
(f) Yang, Z. S.; Li, Y. Chin. J. Org. Chem. 2005, 25, 1342(in Chinese). (杨忠顺, 李英, 有机化学, 2005, 25, 1342.)
(g) Kulinkovich, O. Comprehensive Organic Synthesis Ⅱ, 2nd ed., Elsevier Ltd., Netherlands, 2014, 124.
[10] Weidmann, B.; Seebach, D. Helv. Chim. Acta 1980, 63, 2451.
[11] Herman, D. F.; Nelson, W. K. J. Am. Chem. Soc. 1952, 74, 2693.
[12] Tolstikov, G. A.; Kasatkin, A. N. Izv. Akad. Nauk SSSR, Ser. Khim. 1984, 2835
[13] Kasatkin, A. N.; Tolstikov, G. A.; Kulak, A. N. Izv. Akad. Nauk SSSR, Ser. Khim. 1986, 11, 2527.
[14] (a) Kasatkin, A. N.; Tolstikov, G. A.; Kulak, A. N. Izv. Akad. Nauk SSSR, Ser. Khim. 1987, 2, 391.
(b) Kasatkin, A. N.; Tolstikov, G. A.; Kulak, A. N. Izv. Akad. Nauk SSSR, Ser. Khim. 1988, 9, 2159.
[15] (a) Weber, B.; Seebach, D. Tetrahedron 1994, 50, 7473.
(b) Kim, Y. H.; Byun, I. S.; Choi, J. Y. Tetrahedron:Asymmetry 1995, 6, 1025.
(c) Zhou, S.; Chen, C.-R.; Gau, H.-M. Org. Lett. 2009, 12, 48.
(d) Wu, K.-H.; Zhou, S.; Chen, C.-A.; Yang, M.-C.; Chiang, R.-T.; Chen, C.-R.; Gau, H.-M. Chem. Comm. 2011, 4, 11668.
[16] Han, J. W.; Tokunaga, N.; Hayashi, T. Synlett 2002, 871.
[17] Manolikakes, G.; Dastbaravardeh, N.; Knochel, P. Synlett 2007, 2077.
[18] Lee, H. W.; Lam, F. L.; So, C. M.; Lau, C. P.; Chan, A. S. C.; Kwong, F. Y. Angew. Chem., Int. Ed. 2009, 48, 7436.
[19] (a) Yang, H.-T.; Zhou, S.; Chang, F.-S.; Chen, C.-R.; Gau, H.-M. Organometallics 2009, 28, 5715.
(b) Chen, C.-R.; Zhou, S.; Biradar, D. B.; Gau, H.-M. Adv. Synth. Catal. 2010, 352, 1718.
[20] Chang, S.-T.; Li, Q.-H.; Chiang, R.-T.; Gau, H.-M. Tetrahedron 2012, 68, 3956.
[21] Li, Q.-H.; Liao, J.-W.; Huang, Y.-L.; Chiang, R.-T.; Gau, H.-M. Org. Biomol. Chem. 2014, 12, 7634.
[22] Sato, F.; Urabe, H.; Okamoto, S. Chem. Rev., 2000, 100, 2835.
[23] Harada, K.; Urabe, H.; Sato, F. Tetrahedron Lett. 1995, 36, 3203.
[24] Obora, Y.; Moriya, H.; Tokunaga, M.; Tsuji, Y. Chem. Commun. 2003, 2820.
[25] You, X.; Xie, X.; Sun, R. H.; Chen, H. Y.; Li, S; Liu, Y. H. Org. Chem. Front. 2014, 1, 940.
[26] Rassadin, V. A.; Nicolasb, E.; Six, Y. Chem. Commun. 2014, 50, 7666.
[27] Wittig, G. Angew. Chem. 1958, 70, 65.
[28] Jana, R.; Pathak, T. P.; Sigman, M. S. Chem. Rev. 2012, 111, 1417.
[29] (a) Hatakeyama, T.; Hashimoto, T.; Kondo, Y.; Fujiwara, Y.; Seike, H.; Takaya, H.; Tamada, Y.; Ono, T.; Nakamura, M. J. Am. Chem. Soc. 2010, 132, 10674.
(b) Hashimoto, T.; Hatakeyama, T.; Nakamura, M. J. Org. Chem. 2012, 77, 1168.
(c) Hatakeyama, T.; Hashimoto, T.; Kathriarachchi, K. K. A. D. S.; Zenmyo, T.; Seike, H.; Nakamura, M. Angew. Chem., Int. Ed. 2012, 124, 1.
(d) Nakagawa, N.; Hatakeyama, T.; Nakamura, M. Chem. Lett. 2015, 44, 486.
[30] (a) Muramatsu, Y.; Harada, T. Angew. Chem., Int. Ed. 2008, 47, 1088.
(b) Muramatsu, Y.; Harada, T. Chem.-Eur. J. 2008, 14, 10560.
(c) Nakagawa, Y.; Muramatsu, Y.; Harada, T. Eur. J. Org. Chem. 2010, 34, 6535.
(d) Itakura, D.; Harada, T. Synlett 2011, 2875.
[31] Mikami, K.; Murase, T.; Itoh, Y. J. Am. Chem. Soc. 2007, 129, 11686.
[32] Arai, M.; Nakamura, E. J. Org. Chem. 1991, 56, 5489.
[33] (a) Li, Y.-X.; Xuan, Q.-Q.; Liu, L.; Wang, D.; Chen, Y.-J.; Li, C.-J. J. Am. Chem. Soc. 2013, 135, 12536.
(b) Semba, K.; Nakao, Y. J. Am. Chem. Soc. 2014, 136, 7567.
(c) Labre, F.; Gimbert, Y.; Bannwarth, P.; Olivero, S.; Duñach, E.; Chavant, P. Y. Org. Lett. 2014, 16, 2366.
(d) Seth, K.; Purohit, P.; Chakraborti, A. K. Org. Lett. 2014, 2334.
[34] (a) Mulvey, R. E. Organometallics 2006, 25, 1060.
(b) Mulvey, R. E.; Mongin, F.; Uchiyama, M.; Kondo, Y. Angew. Chem., Int. Ed. 2007, 46, 3802.
[35] Zeng, J.; Liu, K.-M.; Duan, X.-F. Org. Lett. 2013, 15, 5342.
[36] Wei, J.; Liu, K.-M.; Duan, X.-F. J. Org. Chem. 2017, 82, 1291.
[37] (a) Bedford, R. B.; Hall, M. A.; Hodges, G. R.; Huwe, M.; Wilkinson, M. C. Chem. Commun. 2009, 42, 6430.
(b) O'Brien, H. M.; Manzotti, M.; Abrams, R. D.; Elorriaga, D.; Sparkes, H. A.; Davis, S. A.; Bedford, R. B. Nat. Catal. 2018, 1, 429.
[38] Zhang, R.; Zhao, Y.; Liu, K.-M.; Duan, X.-F. Org. Lett. 2018, 7942.
[39] Liu, K.-M.; Liao, L.-Y.; Duan, X.-F. Chem. Commun. 2015, 51, 1124.
[40] Liu, K.-M.; Wei, J.; Duan, X.-F. Chem. Commun. 2015, 51, 4655.
[41] Liao, L.-Y.; Liu, K.-M.; Duan, X.-F. J. Org. Chem. 2015, 80, 9856.
[42] (a) Goossen, L. J.; Paetzold, J.; Winkel, L. Synlett 2002, 1721.
(b) Goossen, L. J.; Deng, G.; Levy, L. M. Science 2006, 313, 662.
(c) Goossen, L. J.; Rodriguez, N.; Melzer, B. J. Am. Chem. Soc. 2007, 129, 4824.
(d) Zhou, J.; Peng, H.; Zhang, M.; Huang, S. J.; Wang, M.; Su, W.-P. Chem.-Eur. J. 2010, 16, 5876.
[43] Liu, K.-M.; Zhang, R.; Duan, X.-F. Org. Biomol. Chem. 2016, 14, 1593.
[44] (a) Lumbroso, A.; Abermil, N.; Breit, B. Chem. Sci. 2012, 3, 789.
(b) Iitsuka, T.; Schaal, P.; Hirano, K.; Satoh, T.; Bolm, C.; Miura, M. J. Org. Chem. 2013, 78, 7216.
(c) Zhang, M. L.; Zhang, H.-J.; Han, T. T.; Ruan, W. Q.; Wen, T.-B. J. Org. Chem. 2015, 80, 620.
[45] (a) Vilardo, J. S.; Thorn, M. G.; Fanwick, P. E.; Rothwell, I. P. Chem. Commun. 1998, 22, 2425.
(b) Turner, L. E.; Thorn, M. G.; Swartz Ⅱ, R. D.; Chesnut, R. W.; Fanwick, P. E.; Rothwell, I. P. Dalton Trans. 2003, 24, 4580.
[46] (a) Boyle, T. J.; Ottley, L. A. M.; Rodriguez, M. A.; Sewell, R. M.; Alam, T. M.; McIntyre, S. K. Inorg. Chem. 2008, 47, 10708.
(b) Muna, S.-D.; Kim, S.-H.; Lee, J.; Kim, H.-J.; Do, Y.-K.; Kim, Y.-J. Polyhedron 2010, 29, 379.
(c) Deivasagayam, D.; Peruch, F. Polymer 2011, 52, 4686.
/
| 〈 |
|
〉 |