

Chinese Journal of Organic Chemistry >
Synthesis and Biological Activities of Novel N-Substitutedphenyl-2-pyrazolylnicotinamides
Received date: 2018-10-19
Revised date: 2018-12-03
Online published: 2019-01-18
Supported by
Project supported by the National Key Research and Development Program of China (Nos. 2017YFD0200505, 2018YFD0200100), the National Natural Science Foundation of China (No. 21772103) and the Natural Science Foundation of Tianjin City (No. 17JCYBJC19900).
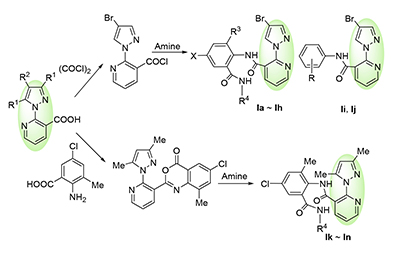
Based on the agrochemical structure of anthranilic diamides, fourteen novel N-substitutedphenyl-2-pyrazolyl- nicotinamides were conveniently synthesized with 2-chloro-3-cyanopyridine and 4-bromopyrazole or 3,5-dimethylpyrazole as starting materials, via an "acyl transposing" design strategy. Their structures were characterized by 1H NMR, 13C NMR and HRMS. The preliminary bioassay tests indicated that most of these compounds exhibited obvious insecticidal activity at the test concentration of 200 mg/L, among which N-(4-chloro-2-(ethylcarbamoyl)-6-methylphenyl)-2-(3,5-dimethyl-1H-pyra- zol-1-yl)nicotinamide (Il) possessed a 70% mortality rate against Mythimna separata Walker; some of the compounds displayed favorable fungicidal activities at 50 mg/L towards Physalospora piricola and Alternaria solani Sorauer, especially 2-(4-bromo-1H-pyrazol-1-yl)-N-(2-(cyclopropylcarbamoyl)-4-iodo-6-methylphenyl)nicotinamide (If) and 2-(4-bromo-1H- pyrazol-1-yl)-N-(4-chloro-2-(ethylcarbamoyl)-6-methylphenyl)nicotinamide (Ih) against Physalospora piricola, and 2-(4- bromo-1H-pyrazol-1-yl)-N-(4-chloro-2-(propylcarbamoyl)phenyl)nicotinamide (Id) against Alternaria solani Sorauer had growth inhibitory rates of 62.9%, 54.3% and 54.5%, respectively. These research results provide important reference for the further study of novel 2-pyrazolylnicotinamide derivatives.
Shang Junfeng , Liu Qiaoxia , Wang Baolei , Li Zhengming . Synthesis and Biological Activities of Novel N-Substitutedphenyl-2-pyrazolylnicotinamides[J]. Chinese Journal of Organic Chemistry, 2019 , 39(5) : 1489 -1496 . DOI: 10.6023/cjoc201810025
[1] Selby, T. P.; Lahm, G. P.; Senvenson, T. M. Pest Manage. Sci. 2017, 73, 658.
[2] Lahm, G. P.; Cordova, D.; Barry, J. D. Bioorg. Med. Chem. 2009, 17, 4127.
[3] Tohnishi, M.; Nakao, H.; Furuya, T.; Seo, A.; Kodama, H.; Tsubata, K.; Fujioka, S.; Kodama, H.; Hirooka, T.; Nishimatsu, T. J. Pestic. Sci. 2005, 30, 354.
[4] Chai, B.; Peng Y.; Li, H.; Zhang, H.; Liu, C. Agrochemicals 2009, 48, 13(in Chinese). (柴宝山, 彭永武, 李慧超, 张弘, 刘长令, 农药, 2009, 48, 13.)
[5] Lahm, G. P.; Selby, T. P.; Freudenberger, J. H.; Stevenson, T. M.; Myers, B. J.; Seburyamo, G.; Smith, B. K.; Flexner, L.; Clark, C. E.; Cordova, D. Bioorg. Med. Chem. Lett. 2005, 15, 4898.
[6] Dong, W.; Xu, J.; Xiong, L.; Liu, X.; Li, Z. Chin. J. Chem. 2009, 27, 579.
[7] Sun, N.; Shen, Z.; Zhai, Z.; Han, L.; Weng, J.; Tan, C.; Liu, X. Chin. J. Org. Chem. 2017, 37, 2705(in Chinese). (孙娜波, 沈钟华, 翟志文, 韩亮, 翁建全, 谭成侠, 刘幸海, 有机化学, 2017, 37, 2705.)
[8] Lamberth, C.; Dinges, J. In Bioactive Heterocyclic Compound Classes——Agrochemicals, Eds.:Lamberth, C.; Dinges, J., Wiley- VCH, Weinheim, 2012, pp. 3~20.
[9] Liu, X.-H.; Fang, Y.-M.; Xie, F.; Zhang, R.-R.; Shen, Z.-H.; Tan, C.-X.; Weng, J.-Q.; Xu, T.-M.; Huang, H.-Y. Pest Manage. Sci. 2017, 73, 1900.
[10] Wang, B.-L.; Zhu, H.-W.; Ma, Y.; Xiong, L.-X.; Li, Y.-Q.; Zhao, Y.; Zhang, J.-F.; Chen, Y.-W.; Zhou, S.; Li, Z.-M. J. Agric. Food Chem. 2013, 61, 5483.
[11] Zhang, J.-F.; Xu, J.-Y.; Wang, B.-L.; Li, Y.-X.; Xiong, L.-X.; Li, Y.-Q.; Ma, Y.; Li, Z.-M. J. Agric. Food Chem. 2012, 60, 7565.
[12] Wang, B.; Ma, Y.; Xiong, L.; Li, Z. Chin. J. Chem. 2012, 30, 815.
[13] Wang, B.-L.; Zhu, H.-W.; Li, Z.-M.; Wang, L.-Z.; Zhang, X.; Xiong, L.-X.; Song, H.-B. Pest Manage. Sci. 2018, 74, 726.
[14] Yan, T.; Yu, S.; Liu, P.; Liu, Z.; Wang, B.; Xiong, L.; Li, Z. Chin. J. Chem. 2012, 30, 919.
[15] Clark, D. A.; Lahm, G. P.; Smith, B. K.; Barry, J. D.; Clagg, D. G. Bioorg. Med. Chem. 2008, 16, 3163.
[16] Cheng, L.; Zhang, R.-R.; Han, L.; Tan, C.-X.; Weng, J.-Q.; Xu, T.-M.; Liu, X.-H. J. Heterocycl. Chem. 2018, 55, 2585.
[17] Shi, J.-J.; Ren, G.-H.; Wu, N.-J.; Weng, J.-Q.; Xu, T.-M.; Liu, X.-H.; Tan, C.-X. Chin. Chem. Lett. 2017, 28, 1727.
[18] Dong, W.-L.; Liu, X.-H.; Xu, J.-Y.; Li, Z.-M. J. Chem. Res. 2008, 9, 530.
[19] Wu, C.-C.; Wang, B.-L.; Liu, J.-B.; Wei, W.; Li, Y.-X.; Liu, Y.; Chen, M.-G.; Xiong, L.-X.; Yang, N.; Li, Z.-M. Chin. Chem. Lett. 2017, 28, 1248.
/
| 〈 |
|
〉 |