

Chinese Journal of Organic Chemistry >
Synthesis of Esters via Sodium Carbonate Promoted Oxa-Michael Addition of Acids to α,β-Unsaturated Ketones
Received date: 2018-12-20
Revised date: 2019-01-25
Online published: 2019-02-19
Supported by
Project supported by the National Natural Science Foundation of China (No. 21702162), and the Scientific Research Program Funded by Shaanxi Provincial Education Department (No. 17JK0788).
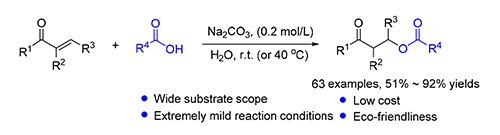
Ester bonds are found widely in various fine chemicals, medicines, pesticides and functional materials. Despite the apparent simplicity, the construction of ester bonds often constitutes the most challenge in the synthesis of complex molecular containing ester functionality. Oxa-Michael addition represents an important class of organic reactions in carbon-heteroatom bond formation. The use of alcohol donors in oxa-Michael additions is well known. However, the use of carboxylic acid as the donor in the reaction is difficult due to the low activity of acid and a more pronounced reversibility of the reaction. To date, there is no general oxa-Michael addition of acids to α,β-unsaturated ketones reported. Herein, an efficient sodium carbonate promoted oxa-Michael addition of acids to α,β-unsaturated ketones to synthesize esters is reported. With a broad substrate scope, a well-common catalyst and simple operation, the approach provides a facile, practicable, economical, and environmentally benign method for the synthesis of esters.
Wang Xingyu , Zhu Xueqing , Jiang Wei , Gao Yaru . Synthesis of Esters via Sodium Carbonate Promoted Oxa-Michael Addition of Acids to α,β-Unsaturated Ketones[J]. Chinese Journal of Organic Chemistry, 2019 , 39(5) : 1383 -1395 . DOI: 10.6023/cjoc201812038
[1] (a) Otera, J. Esterification:Methods, Reactions, and Applications, WileyVCH, Weinheim, 2010.
(b) Morrill, L. C.; Smith, A. D. Chem. Soc. Rev. 2014, 43, 6214.
(c) Majji, G.; Rout, S. K.; Rajamanickam, S.; Guin, S.; Patel, B. K. Org. Biomol. Chem. 2016, 14, 8178.
(d) Mondal, M.; Begum, T.; Bora, U. Org. Chem. Front. 2017, 4, 1430.
[2] Tsakos, M.; Schaffert, E. S.; Clement, L. L.; Villadsen, N. L.; Poulsen, T. B. Nat. Prod. Rep. 2015, 32, 605.
[3] Leth, L. A.; Næsborg, L.; Reyes-Rodríguez, G. J.; Tobiesen, H. N.; Iversen, M. V.; Jørgensen, K. A. J. Am. Chem. Soc. 2018, 140, 12687.
[4] Parenty, A.; Moreau, X.; Niel, G.; Campagne, J. M. Chem. Rev. 2013, 113, PR1.
[5] For selected examples:(a) Fischer, E.; Speier, A. Chem. Ber. 1895, 28, 3252.
(b) Mantri, K.; Komura, K.; Sugi, Y. Green Chem. 2005, 7, 677.
(c) Ishihara, K. Tetrahedron 2009, 65, 1085.
(d) Iranpoor, N.; Firouzabadi, H.; Khalili, D. Org. Biomol. Chem. 2010, 8, 4436.
(e) Nowrouzi, N.; Mehranpour, A. M.; Rad, J. A. Tetrahedron 2010, 66, 9596.
(f) Liu, B.; Hu, F.; Shi, B.-F. ACS Catal. 2015, 5, 1863.
[6] (a) Nising, C. F.; Bräse, S. Chem. Soc. Rev. 2008, 37, 1218.
(b) Jiang, F.; Yuan, F.-R.; Jin, L.-W.; Mei, G.-J.; Shi, F. ACS Catal. 2018, 8, 10234.
(c) Jiang, F.; Luo, G.-Z.; Zhu, Z.-Q.; Wang, C.-S.; Mei, G.-J.; Shi, F. J. Org. Chem. 2018, 83, 10060.
(d) Jiang, F.; Zhao, D.; Yang, X.; Yuan, F.-R.; Mei, G.-J.; Shi, F. ACS Catal. 2017, 7, 6984.
(e) Hong, Y.; Shen, Z.; Mo, W.; Hu, X. Chin. J. Org. Chem. 2009, 29, 1544(in Chinese). (洪一鸣, 沈振陆, 莫卫民, 胡信全, 有机化学, 2009, 29, 1544.)
(f) Ying, A.; Wu, C.; Fu, Y.; Ren, S.; Liang, H. Chin. J. Org. Chem. 2012, 32, 1587(in Chinese). (应安国, 武承林, 付永前, 任世斌, 梁华定, 有机化学, 2012, 32, 1587.)
(g) Li, Z.; Hou, H.; Ying, A.; Xu, S. Chin. J. Org. Chem. 2014, 34, 1074(in Chinese). (李志峰, 侯海亮, 应安国, 许松林, 有机化学, 2014, 34, 1074.)
[7] For select examples:(a) Stewart, I. C.; Bergman, R. G.; Toste, F. D. J. Am. Chem. Soc. 2003, 125, 8696.
(b) Wabnitz, T. C.; Spencer, J. B. Org. Lett. 2003, 5, 2141.
(c) Vanderwal, C. D.; Jacobsen, E. N. J. Am. Chem. Soc. 2004, 126, 14724.
(d) Wabnitz, T. C.; Yu, J.-Q.; Spencer, J. B. Chem. Eur. J. 2004, 10, 484.
(e) Murtagh, J. E.; McCooey, S. H.; Connon, S. J. Chem. Commun. 2005, 227.
(f) Sundén, H.; Ibrahem, I.; Zhao, G.-L.; Eriksson, L.; Córdova, A. Chem. Eur. J. 2007, 13, 574.
(g) Bertelsen, S.; Dinér, P.; Johansen, R. L.; Jørgensen, K. A. J. Am. Chem. Soc. 2007, 129, 1536.
(h) Li, H.; Wang, J.; E-Nunu, T.; Zu, L.; Jiang, W.; Wei, S.; Wang, W. Chem. Commun. 2007, 507.
(i) Xiong, X.; Ovens, C.; Pilling, A. W.; Ward, J. W.; Dixon, D. J. Org. Lett. 2008, 10, 565.
(j) Reyes, E.; Talavera, G.; Vicario, J. L.; Badía, D.; Carrillo, L. Angew. Chem., Int. Ed. 2009, 48, 5701.
(k) Bhuvaneswari, S.; Jeganmohan, M.; Cheng, C.-H. Chem. Asian J. 2010, 5, 141.
(l) Phillips, E. M.; Riedrich, M.; Scheidt, K. A. J. Am. Chem. Soc. 2010, 132, 13179.
[8] (a) Arbuzov, Y. A.; Volkov, Y. P. Z. Obshch. Khim. 1959, 29, 3279.
(b) Hess, H.-J. J. Org. Chem. 1962, 27, 1096.
(c) Weisleder, D.; Friedman, M. J. Org. Chem. 1968, 33, 3542.
(d) Kirchanov, A. A.; Zanina, A. S.; Kotlyarevskii, I. L. Izv. Akad. Nauk SSSR, Ser. Khim. 1981, 8, 1914.
(e) Hofstraat, R. G.; Lange, J.; Scheeren, H. W.; Nivard, R. J. F. J. Chem. Soc. Perkin Trans 1 1988, 2315.
(f) Hosokawa, T.; Shinohara, T.; Ooka, Y.; Murahashi, S.-I. Chem. Lett. 1989, 2001.
(g) Itoh, K.; Utsukihara, T.; Funayama, K.; Sakamaki, H.; Kanamori, M.; Takahashi, T. T.; Saitoh, Y.; Matsushita, M.; He, L.; Hashimoto, C.; Sugiyama, T.; Horiuchi, C. A. Appl. Organomet. Chem. 2007, 21, 1029.
(h) Jha, A. K.; Inani, H.; Easwar, S. Synlett 2017, 28, 1473.
[9] (a) Rinehart, K. L., Jr.; Gloer, J. B.; Hughes, R. G., Jr.; Renis, H. E.; McGovren, J. P.; Swynenberg, E. B.; Stringfellow, D. A.; Kuentzel, S. L. Science 1981, 212, 933.
(b) Ueda, H.; Nakajima, H.; Hori, Y.; Fujita, T.; Nishimura, M.; Goto, T.; Okuhara, M. J. Antibiot.1994, 47, 301.
(c) Randazzo, A.; Bifulco, G.; Giannini, C.; Bucci, M.; Debitus, C.; Cirino, G.; Gomez-Paloma, L. J. Am. Chem. Soc. 2001, 123, 10870.
(d) Walker, S.; Chen, L.; Hu, Y.; Rew, Y.; Shin. D.; Boger, D. L. Chem. Rev. 2005, 105, 449.
(e) Xu, Y.; Kersten, R. D.; Nam, S.-J.; Lu, L.; Al-Suwailem, A. M.; Zheng, H.; Fenical, W.; Dorrestein, P. C.; Moore, B. S.; Qian, P.-Y. J. Am. Chem. Soc. 2012, 134, 8625.
(f) Lam, H. Y.; Zhang, Y.; Liu, H.; Xu, J.; Wong, C. T. T.; Xu, C.; Li, X. J. Am. Chem. Soc. 2013, 135, 6272.
[10] Wrigglesworth, J. W.; Cox, B.; Lloyd-Jones, G. C.; Booker-Miburn, K. I. Org. Lett. 2011, 13, 5326.
[11] Lam, H. W.; Joensuu, P. M. Org. Lett. 2005, 7, 4225.
[12] Iqbal, J.; Srivastva, R. R. J. Org. Chem. 1992, 57, 2001.
/
| 〈 |
|
〉 |