

Chinese Journal of Organic Chemistry >
Application of Chiral 1,1'-Bi-2-naphthol Polymers in Asymmetric Epoxidation of (E)-α,β-Unsaturated Aryl Ketones
Received date: 2018-10-24
Revised date: 2019-01-16
Online published: 2019-03-08
Supported by
Project supported by the National Natural Science Foundation of China (No. 21602188), the Education Department of Hunan Province (No. 17C1524) and the Doctoral Research Initiation Fund of Xiangtan University (No. 15QDZ52).
Most small chiral molecule catalysts are suffered from a rigid process in both product separation and recovery. Therefore more attention has been drawn to the soluble polymer-supported catalyst which could be easily recycled. In this paper, a new type of reusable chiral binaphthol polymer-supported diethylzinc catalyst was synthesized and applied in the asymmetric epoxidation of (E)-α,β-unsaturated aryl ketones. The scope of this reaction was explored. Various (E)-α,β-aryl ketones could be easily prepared in good yield (up to 88%) and high ee value (up to 94%) via this asymmetric epoxidation process. Ligands were recovered to explore the inductive effect of the reaction. Recovery experiments of this binaphthol polymer-supported diethylzinc catalyst were conducted. The results indicate that the asymmetric induction ability of the reclaimed chiral polymer 1,1'-bi-2-naphthol did not decrease significantly.
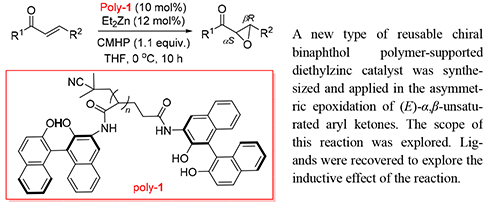
Key words: 1,1'-bi-2-naphthol; Et2Zn; chiral polymer; asymmetric addition; aromatic ketone
Cai Han , Ouyang Kunbing , Yang Nianfa . Application of Chiral 1,1'-Bi-2-naphthol Polymers in Asymmetric Epoxidation of (E)-α,β-Unsaturated Aryl Ketones[J]. Chinese Journal of Organic Chemistry, 2019 , 39(5) : 1456 -1459 . DOI: 10.6023/cjoc201810029
[1] Lauret, C. Tetrahedron:Asymmetry 2001, 12, 2359.
[2] Bougauchi, M.; Watanabe, S.; Arai, T.; Sasai, H.; Shibasaki, M. J. Am. Chem. Soc. 1997, 119, 2329.
[3] Wang, X. W.; Shi, L.; Li, M. X.; Ding, K. L. Angew. Chem., Int. Ed. 2005, 117, 6520.
[4] Ooi, T.; Ohara, D.; Tamura, M.; Maruoka, K. J. Am. Chem. Soc. 2004, 126, 6844.
[5] Yi, H.; Zou, G.; Li, Q.; Chen, Q.; Tanga, J.; He, M. Y. Tetrahedron Lett. 2005, 46, 5665.
[6] Daikai, K.; Kamaura, M.; Inanaga, J. Tetrahedron Lett. 1998, 39, 7321.
[7] Lattanzi, A. Adv. Synth. Catal. 2006, 348, 339.
[8] Minatti A.; Dötz K. H. Eur. J. Org. Chem. 2006, 1, 268.
[9] (a) Liu. D. C.; Ouyang. K. B.; Yang. N. F. Tetrahedron 2016, 72, 1018.
(b) Zhang. A. L.; Yu. Z. D.; Yang. L. W.; Yang. N. F. Tetrahedron:Asymmetry 2015, 26, 173.
(c) Zhang. A. L.; Yu. Z. D.; Yang. L. W.; Yang. N. F.; Peng. D. J. Mol. Catal. A:Chem. 2015, 398, 407.
[10] Sellner. H.; Faber. C.; Rheiner. P. B.; Seebach. D. Chem.-Eur. J. 2000, 6, 3692.
[11] Fleischmann. C.; Ritter, H. Polym Int. 2015, 64, 724.
[12] Zhang, X.; Yang, N. F. Chin. J. Org. Chem. 2017, 37, 1027(in Chinese). (张勋, 阳年发, 有机化学, 2017, 37, 1027.)
[13] Wang, B.; Wang, S.; Xia, C.; Sun, W. Chem.-Eur. J. 2012. 18, 7332.
[14] Lv, J.; Wang, X.; Liu, J.; Zhang, L.; Wang, Y. Tetrahedron:Asymmetry 2006, 17, 330.
[15] Yoo, M. S.; Kim, D. G.; Ha, M. W.; Jew, S. S.; Park, H. G.; Jeong, B. S. Tetrahedron Lett. 2010, 51, 5601.
/
| 〈 |
|
〉 |