

Chinese Journal of Organic Chemistry >
Synthesis and Biological Activity of Thiazolidine Piperidine Nicotinamide Compounds
Received date: 2018-12-14
Revised date: 2019-02-18
Online published: 2019-03-08
Supported by
Project supported by the Collaborative Innovation Center of Zhejiang Province Green Pesticide.
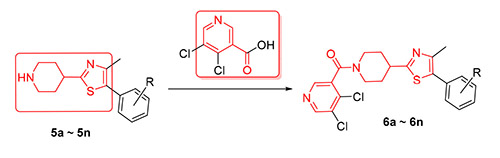
The structure of thiazolidine piperidine could affect the metabolic activitives of cholesterol compounds in vivo, and it is the key pharmacophore of oxythiazolidine to inhibit the oxygen cholesterol-binding protein (OSBP) of pathogenic bacteria. 14 novel thiazolidine piperidine nicotinamide derivatives were designed and synthesized in search of new bioactive compounds containing thiazolidine piperidine structure. The structures of target compounds were characterized by 1H NMR, 13C NMR and HRMS spectra. The preliminary bioassay showed that the target compounds generally had antibacterial activities. At the concentration of 100 μg/mL, the antibacterial activity of one compound against Fusarium graminearum was 60%, the antibacterial activities of three compounds against Botrytis cinerea were 60%, the bacteriostatical activities of six compounds aganist Diplocarpon mali were 70%, and the antibacterial activity of (4-(5-(2,3-dichlorophenyl)-4-methylthiazol-2- yl)piperidin-1-yl)(5,6-dichloropyridin-3-yl)methanone (6k) against Phytophthora infestans (Mont.) de Bary was 75%. There- fore it was worth for further research about structural optimization.
Key words: structure; thiazolidine piperidine; nicotinamide; antibacterial activity
Ding Chengrong , Pan Yayun , Yin Xu , Tan Chengxia . Synthesis and Biological Activity of Thiazolidine Piperidine Nicotinamide Compounds[J]. Chinese Journal of Organic Chemistry, 2019 , 39(7) : 2099 -2105 . DOI: 10.6023/cjoc201812027
[1] Huang, X. F.; Tang, J. F.; Ji, J. L. J. Organomet. Chem. 2012, 878, 1.
[2] Chavva, K.; Pillalamarri, S.; Banda, V. Bioorg. Med. Chem. Lett, 2014, 23, 5893.
[3] Dev, G. J.; Poornachandra, Y.; Reddy, K. R.; Kumar, R. N. Eur. J. Med. Chem. 2017, 130, 223.
[4] Liu, R. B.; Zheng, Y. Q.; Wang, Y. L. Agrochemicals 2018, 57, 326(in Chinese). (刘瑞宾, 郑怡倩, 汪焱鲁, 农药, 2018, 57, 326.)
[5] Cederbaum, F. WO 2014118142, 2014[Chem. Abstr. 2014, 161, 320959].
[6] Lamberth, C. WO 2014154530, 2014[Chem. Abstr. 2014, 161, 640429].
[7] Tomoki, T. US 20130296272, 2013[Chem. Abstr. 2014, 156, 337332].
[8] Gregory, V. WO 2009055514, 2009[Chem. Abstr. 2009, 150, 578441].
[9] Stefan, H. US 20160198713, 2016[Chem. Abstr. 2016, 162, 400022].
[10] Pierre, C. US 20110312999, 2011[Chem. Abstr. 2011, 155, 615369].
[11] Hanagan, M. A. WO 2009094407, 2009[Chem. Abstr. 2009, 151, 234956].
[12] Kamireddy, B. WO 2009094445, 2009[Chem. Abstr. 2009, 151, 173451].
[13] Pasteris, R. J. WO 2008013925, 2008[Chem. Abstr. 2008, 160, 302215].
[14] Hu, D. J.; Liu, S. F.; Huang, T. H. Molecules 2009, 14, 1288.
[15] Chen, L.; Zhu, Y. J.; Fan, Z. J. J. Agric. Food Chem. 2017, 65, 745.
[16] Sulzermosse, S.; Cederbaum, F.; Lamberth, C. Bioorg. Med. Chem. 2015, 23, 2129.
[17] Choi, W. S.; Nam, S. W.; Kim, I. D.; Kim, S. H.; Park, K. H.; Bae, I. K. J. Chem. 2015, 241793.
[18] Li, F. Y.; Zhu, Y. J.; Fan, Z. J.; Xu, J. H.; Guo X. F.; Zong, G. N.; Song, Y. Q.; Qian, X. L.; Ma, L. Y.; Wang, J. R. Chin. J. Struct. Chem. 2015, 34, 5659.
[19] Wang, K. B.; Pu, T.; Luo, Y. J. Yunnan Agric. Univ. 2018, 33, 218(in Chinese). (王凯博, 普特, 罗艳, 云南农业大学学报, 2018, 33, 218.)
[20] Ye, X. World Pestic. 2018, 40, 63(in Chinese). (叶萱. 世界农药, 2018, 40, 63.)
[21] Xu, J.; Ma, L.; Liu, X. B. Chin. J. Org. Chem. 2018, 38, 1680(in Chinese). (徐姣, 马玲, 刘秀波, 有机化学, 2018, 38, 1680.)
[22] Sun, N. B.; Shen, Z. H.; Zhai, Z. W. Chin. J. Org. Chem. 2017, 37, 2044(in Chinese). (孙娜波, 沈钟华, 翟志文, 有机化学, 2017, 37, 2044.)
[23] Dai, H.; Li, H.; Jin, Z. C. Chin. J. Org. Chem. 2016, 36, 185(in Chinese). (戴红, 李宏, 金智超, 有机化学, 2016, 36, 185.)
[24] Dai, H.; Chen, J.; Cao, X. F. Chin. J. Org. Chem. 2016, 36, 377(in Chinese). (戴红, 陈佳, 曹雄飞, 有机化学, 2016, 36, 377.)
[25] Li, Q. M.; Pang, K. S.; Zhao, J. P. Chin. J. Org. Chem. 2017, 37, 1009(in Chinese). (李倩梅, 庞凯胜, 赵建平, 有机化学, 2017, 37, 1009.)
[26] Li, L.; Chen, H.; Lin, Y. Synth. Commun. 2007, 37, 85.
[27] Groß, A.; Schneiders, N.; Daniel, K. Tetrahedron 2008, 64, 10882.
/
| 〈 |
|
〉 |