

Chinese Journal of Organic Chemistry >
Synthesis and Biological Activity Study of Novel Cyano-containing Multi-substituted Pyrazoles Obtained via Strecker Reaction
Received date: 2018-12-11
Revised date: 2019-02-28
Online published: 2019-03-21
Supported by
Project supported by the National Natural Science Foundation of China (No. 20902107).
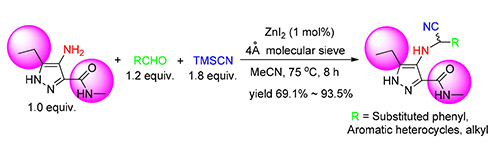
In order to study the Strecker reaction of multi-substituted amino armorotic heterocycles and the biological activity of the target compounds, the reaction of 3,5-disubstituted pyrazole-4-amine, TMSCN and aldehydes was realized for the first time by the catalysis of anhydrous ZnI2 with 4Å molecular seive. The reaction condition was preliminarily optimized and the substrate scop of aldehydes was studied. 20 target compounds of cyano-containing multi-substituted pyrazoles were obtained with the highest yield of 93.5%, and the sturctures of the compounds were confirmed via 1H NMR, 13C NMR and HRMS methods. Priliminary bioassay of the target compounds showed that 13 target compounds possessed 100% larvicidal activity against mosquito at a concentration of 10×10-3 g/L, and four compounds possessed over 40% larvicidal activity at 5×10-3 g/L, and 10 compounds poccessed weak larvicidal activity against army worm at 500×10-3 g/L with the highest activity of 40%; five compounds were confirmed poccessed good inactivation activity against tobacco mosaic virus (TMV) in vivo with the highest inhibition rate of 31.8%, and four compounds possessed moderate host-protection activity against TMV in vivo with the highest rate of 28.3%; in addition, at a concentration of 50×10-3 g/L, three compounds showed moderate fungicidal activity against Pythium aphanidermatum, Phomopsis asparagi, Phytophora capsic and Rhizoctonia solani in vitro. 4-((1-cyanododecyl)amino)-5-ethyl-N-methyl-1H-pyrazole-3-carboxamide (3t) possessed the highest activity against Phytophora capsic of 62.3%. All the results showed that 3,5-disubstitued pyrazole-4-amine could undergo Strecker reaction smoothly, and this study gave a useful example to explore the Strecker reaction of other multi-substituted aromatic hetreocyclic amines. Additionally, the larvicidal and anti-TMV activity of the target compounds gave some clues in design cyano-containing biological heterocycles.
Su Shimiao , Zhu Mo , Zhang Daqiang , Yuan Dekai . Synthesis and Biological Activity Study of Novel Cyano-containing Multi-substituted Pyrazoles Obtained via Strecker Reaction[J]. Chinese Journal of Organic Chemistry, 2019 , 39(7) : 2026 -2034 . DOI: 10.6023/cjoc201812019
[1] (a) Strecker, D. Ann. Chem. Pharm. 1850, 75, 27.
(b) Kouznetsov, V. V.; Galvis, C. E. P. Tetrahedron 2018, 74, 773.
[2] (a) de Bruin, G.; Mock, E. D.; Hoogendoorn, S.; van den Nieuwendijk, A. M. C. H.; Mazurek, J.; van der Marel, G. A.; Florea, B. I.; Overkleeft, H. S. Chem. Commun. 2016, 52, 4064.
(b) Hirata, T.; Ueda, A.; Oba, M.; Doi, M.; Demizu, Y.; Kurihara, M.; Nagano, M.; Suemune, H.; Tanaka, M. Tetrahedron 2015, 71, 2409.
[3] (a) Song, R. Z.; Han, Z. M.; He, Q. Q.; Fan, R. H. Org. Lett. 2016, 18, 5328.
(b) Tay, G. C.; Sizemore, N.; Rychnovsky, S. D. Org. Lett. 2016, 18, 3050.
(c) Guchhait, S. K.; Priyadarshani, G.; Gulghane, N. M. RSC Adv. 2016, 6, 56056.
(d) Afraj, S. N.; Chen, C. P.; Lee, G. H. RSC Adv. 2016, 6, 29783.
[4] (a)Yashin, N. V.; Averina, E. B.; Sedenkova, K. N.; Kuznetsova, T. S.; Zefirov, N. S. Russ. Chem. Bull. 2013, 62, 928.
(b) Acena, J. L.; Sorochinsky, A. E.; Soloshonok, V. A. Synthesis 2012, 44, 1591.
[5] (a) Malins, L. R.; deGruyter, J. N.; Robbins, K. J.; Scola, P. M.; Eastgate, M. D.; Ghadiri, M. R.; Baran, P. S. J. Am. Chem. Soc. 2017, 139, 5233.
(b) Xing, J. H.; Brooks, A. F.; Fink, D.; Zhang, H. B.; Piert, M. R.; Scott, P. J. H.; Shao, X. Synlett 2017, 28, 371.
(c) Cai, H. C.; Mangner, T. J.; Muzik, O.; Wang, M. W.; Chugani, D. C.; Chugani, H. T. ACS Med. Chem. Lett. 2014, 5, 1152.
(d) Kokhan, S. O.; Tymtsunik, A. V.; Grage, S. L.; Afonin, S.; Babii, O.; Berditsch, M.; Strizhak, A. V.; Bandak, D.; Platonov, M. O.; Komarov, I. V.; Ulrich, A. S.; Mykhailiuk, P. K. Angew. Chem., Int. Ed. 2016, 55, 14788.
[6] (a) Katsuhiko, M.; Bilal, A. A.; Christopher, M. S.; Shajila, S.; Michio, K. J. Am. Chem. Soc. 2016, 138, 12975.
(b) Dhanasekaran, S.; Suneja, A.; Bisai, V.; Singh, V. K. Org. Lett. 2016, 18, 634.
(c) Chen, C. H.; Genapathy, S.; Fischer, P. M.; Chan, W. C. Org. Biomol. Chem. 2014, 12, 9764.
(d) Aleiwi, B. A.; Schneider, C. M.; Kurosu, M. J. Org. Chem. 2012, 77, 3859.
[7] (a) Brahmachari, G.; Kumar, A.; Srivastava, A. K.; Gangwar, S.; Misra, N.; Gupta, V. K.; Rajnikant, R. RSC Adv. 2015, 5, 80967.
(b) Luis, J. A. S.; De Aquino, T. M.; Lira, B.F.; Filho, P. F. A.; Scotti, M. T.; Scotti, L.; De Moura, R. O.; Mendonca, F. J. Acta Pharm. (Zagreb, Crotia) 2014, 64, 233.
[8] (a) Carreno Otero, A. L.; Vargas Mendez, L. Y.; Duque L., J. E.; Kouznetsov, V. V. Eur. J. Med. Chem. 2014, 78, 392.
(b) Yu, X. H.; Wang, G. H.; Kong, X. L.; Huang, H. Y. CN 103183707, 2013[Chem. Abstr. 2013, 159, 229714].
(c) Lamberth, C.; Dumeunier, R.; Trah, S.; Wendeborn, S.; Godwin, J.; Schneiter, P.; Corran, A. Bioorg. Med. Chem. 2013, 21, 127.
[9] (a) Parker, E. T.; Zhou, M. S.; Burton, A. S.; Glavin, D. P.; Dworkin, J. P.; Krishnamurthy, R.; Fernandez, F. M.; Bada, J. L. Angew. Chem., Int. Ed. 2014, 53, 8132.
(b) Bolm, C.; Mocci, R.; Schumacher, C.; Turberg, M.; Puccetti, F.; Hernandez, J. G. Angew. Chem., Int. Ed. 2018, 57, 2423.
(c) Riffet, V.; Frison, G.; Bouchoux, G. J. Phys. Chem. A 2018, 122, 1643.
[10] Li, P. C.; Zhang, Y. D.; Chen, Z. L.; Zhang, X. X. Tetrahedron Lett. 2017, 58, 1854.
[11] Reddy, V. V. R.; Saritha, B.; Ramu, R.; Varala, R.; Jayashree, A. Asian J. Chem. 2014, 26, 7439.
[12] Hajipour, A. R.; Dehbane, I. M. Iran. J. Catal. 2012, 2, 147.
[13] Wang, S. Y.; Xu, J. N.; Zheng, J. F.; Chen, X. D.; Shan, L.; Gao, L. J.; Wang, L.; Yu, M.; Fan, Y. Inorg. Chim. Acta 2015, 437, 81.
[14] (a) Chai, J.; Wang, P. C.; Jia, J.; Ma, B.; Sun, J.; Tao, Y. F.; Zhang, P.; Wang, L.; Fan, Y. Polyhedron 2018, 141, 369.
(b) Ibanez, S.; Poyatos, M.; Peris, E. Chem. Comm. 2017, 53, 3733.
(c) Prakash, G. K. S.; Mathew, T.; Panja, C.; Kulkarni, A.; Olah, G. A.; Harmer, M. A. Adv. Synth. Catal. 2012, 354, 2163.
[15] (a) Saravanan, S.; Khan, N. H.; Jakhar, A.; Ansari, A.; Kureshy, R. I.; Abdi, S. H. R.; Kumar, G. RSC Adv. 2015, 5, 99951.
(b) Zhu, C.; Xia, J. Bao; Chen, C. Tetrahedron Lett. 2014, 55, 232.
[16] Costantini, N. V.; Bates, A. D.; Haun, G. J.; Chang, N. M.; Moura- Letts, G. ACS Sustainable Chem. Eng. 2016, 4, 1906.
[17] Li, N. B.; Wang, J. Y.; Zhang, X. H.; Qiu, R. H.; Wang, X; Chen, J. Y.; Yin, S. F.; Xu, X. H. Dalton Trans. 2014, 43, 11696.
[18] Brahmachari, G.; Banerjee, B. Asian J. Org. Chem. 2012, 1, 251.
[19] Zhang, X. L.; Wu, Q. P.; Zhang, Q. S. J. Chem. Res. 2013, 37, 690.
[20] Ghasemnejad-Bosra, H.; Arta, R. Org. Chem.: Indian J. 2015, 11, 399.
[21] Huber, R.; Bigler, R.; Mezzetti, A. Organometallics 2015, 34, 3374.
[22] Khalafi-Nezhad, A.; Divar, M.; Panahi, F. J. Org. Chem. 2013, 78, 10902.
[23] Ghafuri, H.; Roshani, M. RSC Adv. 2014, 4, 58280.
[24] Nammalwar, B.; Fortenberry, C.; Bunce, R. A. Tetrahedron Lett. 2014, 55, 379.
[25] (a) Khazdooz, L.; Zarei, A.; Hajipour, A. R.; Sheikhan, N. Iran. J. Catal. 2012, 2, 63.
(b) Akbari, J. C. R. Chim. 2012, 15, 471.
[26] Sengupta, A.; Su, C. L.; Bao, C. L.; Nai, C. T.; Loh, K. P. ChemCatChem 2014, 6, 2507.
[27] Zhang, J.; Du, G. F.; Gu, C. Z.; Dai, B. J. Chin. Org. Chem. 2017, 37, 914(in Chinese). (张洁, 杜广芬, 顾承志, 代斌, 有机化学, 2017, 37, 914.)
[28] (a) Chen, W.; Peng, X. W.; Zhong, L. X.; Li, Y.; Sun, R. C. ACS Sustainable Chem. Eng. 2015, 3, 1366.
(b) Dabral, S.; Turberg, M.; Wanninger, A.; Bolm, C.; Hernandez, J. G. Molecules 2017, 22, 146/1.
[29] Dekamin, M. G.; Azimoshan, M.; Ramezani, L. Green Chem. 2013, 15, 811.
[30] (a) Islami, M.; Dekamin, M. G.; Motlagh, L.; Maleki, A. Green Chem. Lett. Rev. 2018, 11, 36.
(b) Reinares-Fisac, D.; Aguirre-Diaz, L. M.; Iglesias, M.; Snejko, N.; Gutierrez-Puebla, E.; Monge, M. A.; Gandara, F. J. Am. Chem. Soc. 2016, 138, 9089.
(c) Xia, J.; Xu, J. N.; Fan, Y.; Song, T. Y.; Wang, L.; Zheng, J. F. Inorg. Chem. 2014, 53, 10024.
(d) Chen, H.; Ju, J.; Meng, Q. P.; Su, J.; Lin, C.; Zhou, Z.Y.; Li, G. B.; Wang, W. L.; Gao, W. L.; Zeng, C. M.; Tang, C.; Lin, J. H.; Yang, T.; Sun, J. L. J. Am. Chem. Soc. 2015, 137, 7047.
(e) Wang, W. L.; Wang, Y.; Wu, B.; Cong, R. H.; Gao, W. L.; Qin, B.; Yang, T. Catal. Commun. 2015, 58, 174.
[31] (a) Azizi, N.; Farhadi, E. Appl. Organomet. Chem. 2018, 32, e4188.
(b) Arora, P.; Rajput, J. K.; Singh, H. RSC Adv. 2015, 5, 97212.
(c) Indalkar, K. S.; Khatri, C. K.; Chaturbhuj, G. U. Tetrahedron Lett. 2017, 58, 2144.
(d) Mobaraki, A.; Movassagh, B.; Karimi, B. ACS Comb. Sci. 2014, 16, 352.
(e) Gawande, M. B.; Rathi, A. K.; Nogueira, I. D.; Varma, R. S.; Branco, P. S. Green Chem. 2013, 15, 1895.
[32] (a) Chen, D.; Xu, M. H. J. Org. Chem. 2014, 79, 7746.
(b) Dekamin, M. G.; Mokhtari, Z. Tetrahedron 2012, 68, 922.
(c) Yang, K.; Liu, L. J.; Liu, J. T. J. Org. Chem. 2014, 79, 3215.
(d) Yuan, X. M.; Xu, J.; Liu, Z. J.; Yang, X. J.; Wang, L. M.; Zhang, Y.; Yang, X. Y.; He, X. P.; Liu, J. T. J. Fluorine Chem. 2012, 144, 102.
[33] Miao, Z. W. In Tools for Stereoselective Synthesis, Eds.:Martin, M.; Boysen, K., Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2013, Chapter 1, pp. 3~26.
[34] (a) Pellissier, H. Chem. Rev. 2013, 113, 442.
(b) Sashikanth, S.; Raju, V.; Somaiah, S.; Rao, P. S.; Reddy, K. V. Synthesis 2013, 45, 621.
[35] Oliveira, M. T.; Lee, J. W. ChemCatChem 2017, 9, 377.
[36] (a) Sugiishi, T.; Matsugi, M.; Hamamoto, H.; Amii, H. RSC Adv. 2015, 5, 17269.
(b) Sadhukhan, A.; Saravanan, S.; Khan, N. H.; Kureshy, R. I.; Abdi, S. H. R.; Bajaj, H. C. J. Org. Chem. 2012, 77, 7076.
(c) Su, Z. S.; Li, W. Y.; Wang, J.; Hu, C. W.; Feng, X. M. Chem.- Eur. J. 2013, 19, 1637.
(d) Wang, D.; Liang, J. Y.; Feng, J. C.; Wang, K. R.; Sun, Q. T.; Zhao, L.; Li, D.; Yan, W. J.; Wang, R. Adv. Synth. Catal. 2013, 355, 548.
[37] (a) Vesely, J.; Rios, R. Chem. Soc. Rev. 2014, 43, 611.
(b) Liu, Y. L.; Yin, X. P.; Zhou, J. Chin. J. Chem. 2018, 36, 321.
(c) Iwanejko, J.; Wojaczynska, E. Org. Biomol. Chem. 2018, 16, 7296.
[38] Yan, H. L.; Oh, J. S.; Lee, J. W.; Song, C. E. Nat. Commun. 2012, 3, 2216/1.
[39] (a) Kaur, J.; Chimni, S. S. Org. Biomol. Chem. 2018, 16, 3328.
(b) Wang, H. Y.; Zheng, C.W.; Chai, Z.; Zhang, J. X.; Zhao, G. Nat. Commun. 2016, 7, 12720.
[40] (a) Karahan, S.; Tanyeli, C. Tetrahedron Lett. 2018, 59, 3725.
(b) Zhao, B. L.; Li, J. H.; Du, D. M. Chem. Rec. 2017, 17, 1.
[41] Agnew-Francis, K. A.; Williams, C. M. Adv. Synth. Catal. 2016, 358, 675.
[42] Xue, H. S.; Tan, C. H.; Wong, M. W. Can. J. Chem. 2016, 94, 1099.
[43] Agarwal, J. Org. Biomol. Chem. 2016, 14, 10747.
[44] (a) Hatano, M.; Ishihara, K. Asian J. Org. Chem. 2014, 3, 352.
(b) Ma, J. A.; Zhang, G. W. In Tools for Stereoselective Synthesis, Eds.:Martin, M.; Boysen K., Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2013, Chapter 16, pp. 351~370.
[45] Hou, Y. L.; Sun, R. W. Y.; Zhou, X. P.; Wang, J. H.; Li, D. Chem. Commun. 2014, 50, 2295.
[46] Mendoza, J. H. Q.; Henao, J. A.; Otero, A. L. C.; Kouznetsov, V. V. Powder Diffr. 2016, 31, 149.
[47] Martinez-Ariza, G.; Nunez-Rios, J.; Lee, Y. S.; Hulme, C. Tetrahedron Lett. 2015, 56, 1038.
[48] Hu, X. C.; Li, R. Z; Li, Z. J. Chem. Res. 2014, 38, 432.
[49] (a) Hall, R. G.; Edmunds, A.; Jeanguenat, A. WO 2014166795, 2014[Chem. Abstr. 2014, 161, 613546].
(b) Buysse, A. M.; Niyaz, N. M.; Demeter, D. A.; Zhang, Y.; Walsh, M. J.; Kubota, A.; Hunter, R.; Trullinger, T. K.; Lowe, C. T.; Knueppel, D.; Patny, A.; Garizi, N.; Leplae, P. R. J.; Wessels, F.; Ross, R. J.; Deamicis, C.; Borromeo, P. US 20140213448, 2014[Chem. Abstr. 2014, 161, 275906].
(c) Dai, H.; Xiao, Y.-S.; Li, Z.; Xu, X.-Y.; Qian, X.-H. Chin. Chem. Lett. 2014, 25, 1014.
[50] (a) Zhang Y. B. World Pestic. 2013, 35, 38(in Chinese). (张一宾, 世界农药, 2013, 35, 38.)
(b) Moffat A. S. Science 1993, 261, 550.
(c) Loso, M. R.; Nugent, B. M.; Huang, J. X.; Rogers, R. B.; Zhu, Y.; Renga, J. M.; Hegde, V. B.; Demark, J. J. WO 2007095229, 2007[Chem. Abstr. 2007, 147, 270793].
(d) Manabe, A.; Enomoto, M.; Yamada, Y.; Oguri, Y.; Sasaki, M. Pestic. Sci. 1999, 55, 649.
[51] (a) Zhang, D. Q.; Xu, G. F.; Liu, Y. H.; Wang, D. Q.; Yang, X. L.; Yuan, D. K. Chin. J. Org. Chem. 2015, 35, 2191(in Chinese). (张大强, 徐高飞, 刘艳红, 王道全, 杨新玲, 袁德凯, 有机化学, 2015, 35, 2191.)
(b) Zhang, D. Q.; Xu, G. F.; Fan, Z. J.; Wang, D. Q.; Yang, X. L.; Yuan, D. K. Chin. Chem. Lett. 2012, 23, 669.
[52] Xu, G. F.; Liu, Y. H.; Yang, X. L.; Wang D. Q.; Yuan, D. K. Chem. J. Chin. Univ. 2016, 37, 486(in Chinese). (徐高飞, 刘艳红, 杨新玲, 王道全, 袁德凯, 高等学校化学学报, 2016, 37, 486.)
/
| 〈 |
|
〉 |