

Chinese Journal of Organic Chemistry >
Synthesis of 8-Prenylflavonoids Natural Products by Microwave Promoted Claisen Rearrangement
Received date: 2018-12-11
Revised date: 2019-03-01
Online published: 2019-03-29
Supported by
Project supported by the Key Laboratory of Hunan Province for Fruit and Vegetable Processing and Quality Safety (No. 2108TP1030) and the Hunan Provincial Natural Science Foundation of China (No. 2018JJ2034).
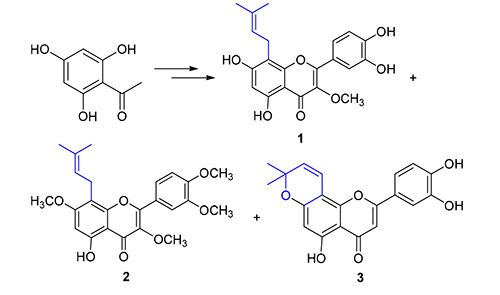
8-Prenylflavonoids are a class natural products with significant biological activities. The total synthesis of three prenylflavonoid natural products, 8-prenylquercetin-3-methylether (1), 8-prenylquerccetin-3,7,3',4'-tetramethyl ether (2) and Artochamin C (3), was achieved through methoxymethyl protection, aldol condensation, iodine catalytic cyclization, dimethyl sulfoxide (DMSO) oxidation, O-prenylation, microwave promoted Claisen rearrangement, deprotection, O-methylation and prenyl group side chain cyclization, staring from commercially available 2,4,6-trihydroxyacetophenone and 3,4-dihydroxy benzaldehyde. The key step of microwave promoted Claisen rearrangement formed 8-C-prenylflavonoids from 5-O- prenylflavonoids was investigated. All the synthesized compounds were confirmed by 1H NMR、13C NMR and MS techniques.
Li Wei , Shu Liang , Wang Qiuan , Li Gaoyang , Shan Yang . Synthesis of 8-Prenylflavonoids Natural Products by Microwave Promoted Claisen Rearrangement[J]. Chinese Journal of Organic Chemistry, 2019 , 39(7) : 1976 -1982 . DOI: 10.6023/cjoc201812020
[1] Dong, X. W.; Liu, Y. J.; Yan, J. Y.; Jiang, C. Y.; Chen, J.; Liu, T.; Hu, Y. Z. Bioorg. Med. Chem. 2008, 16, 8151.
[2] Paoletti, T.; Fallarini, S.; Gugliesi, F.; Minassi, A.; Appendino, G.; Lombardi, G. Eur. J. Pharmacol. 2009, 620, 120.
[3] Daskiewicz, J. B.; Depeint, F.; Viornery, L.; Bayet, C.; Comte-Sarrazin, G.; Comte, G.; Barron, D. J. Med. Chem. 2005, 48, 2790.
[4] Shang, M. Y.; Wang, Q. H.; Xiao, J. J. CN 102382092, 2012. Pinhey, J.T.; Southwell, I. A. Austr. J. Chem. 1973, 26, 409.
[5] Wang, Y. H.; Hou, A.J.; Chen, L.; Chen, D. F.; Sun, H. D.; Zhao, Q. S.; Bastow, K. F.; Nakanish, Y.; Wang, X. H.; Lee, K. H. J. Nat. Prod. 2004, 67, 757.
[6] Wang, Z. Q.; Wang, H. H.; Wu, J. Y. Chem. Biol. Interact. 208, 179, 375.
[7] Zeng, L.; Fukai, T.; Nomura, T.; Zhang, R. Y.; Lou, Z. C. J. Chem. Soc., Perkin Trans. 1 1993, 1153.
[8] Majumdar, K. C.; Nandi, R. K. Tetrahedron 2013, 69, 6921.
[9] Daskiewicz, J. B.; Bayet, C.; Barron, D. Tetrahedron Lett. 2001, 42, 7241.
[10] Al-Maharik, N.; Botting, N. P. Tetrahedron 2003, 59, 4177.
[11] Mu, G. M.; Pu, W. C.; Zhou, M.; Liu, Y.; Yang, H. J.; Wang, C. Chin. J. Org. Chem. 2013, 33, 1298(in Chinese). (牟关敏, 蒲文臣, 周敏, 刘燕, 杨海君, 王淳, 有机化学, 2013, 33, 1298.)
[12] Lan, C.; Xu, P.; Liang, R. H.; Xu, Z. L.; Xia, Z. N. Chin. J. Org. Chem. 2012, 32, 765(in Chinese). (兰聪, 徐盼, 梁荣辉, 许泽龙, 夏之宁, 有机化学, 2012, 32, 765.)
[13] Nguyen, V. S.; Dong, L. P.; Wang, S. C.; Wang, Q. A. Eur. J. Org. Chem. 2015, 10, 2297.
[14] Wu, Z.; Cai, S. L.; Fan, W. J.; Wang, Q. A. Chin. J. Org. Chem. 2012, 32, 1296(in Chinese). (吴峥, 蔡双莲, 范文金, 汪秋安, 有机化学, 2012, 32, 1296.)
[15] Detsi, A.; Majdalani, M.; Kontogiorgis, C. A.; Hadjipavlou-Litina, D.; Kefalas, P. Bioorg. Med. Chem. 2009, 17, 8073.
/
| 〈 |
|
〉 |