

Chinese Journal of Organic Chemistry >
Design, Synthesis and Evaluation of Anti-tumor Activities of Chidamide Derivatives
Received date: 2018-12-19
Revised date: 2019-01-12
Online published: 2019-03-29
Supported by
Project supported by the Key Research and Development Plan of Shandong Province (No. 2017CXGC1401).
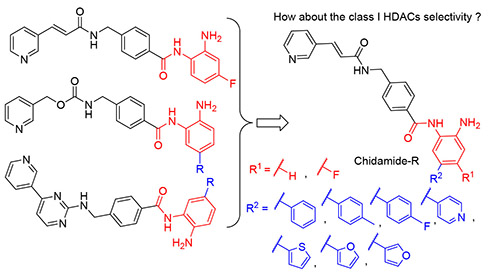
A series of novel chidamide based histone deacetylases (HDACs) inhibitors were rationally designed and synthesized to increase the Zn2+ chelating and selectivity. Biological characterization established that most of the compounds showed moderate antiproliferative activitites in cancer cell lines. Among the tested analogs, (E)-N-(4-amino-6- fluoro-[1,1'-biphenyl]-3-yl)-4-((3-(pyridin-3-yl)acrylamido)methyl)benzamide (7i) and (E)-N-(2-amino-4-fluoro-5-(thiophen- 2-yl)phenyl)-4-((3-(pyridin-3-yl)acrylamido)methyl)benzamide (7j) exhibit the most potent antiproliferative activity with IC50 of 3.29 and 2.59 μmol/L in Jurkat cells, respectively. Furthermore, these two compounds have a certain HDAC inhibitory activity. Collectively, the results partly encourage further development of more potential analogs based on chidamide.
Key words: chidamide; HDACs; anti-tumor activity; benzamide
Zhang Xiangna , He Feng , Zhang Qiuqiong , Lü Jiahui , Xu A'na , Yu Chenggong , Qu Ying , Wu Jingde . Design, Synthesis and Evaluation of Anti-tumor Activities of Chidamide Derivatives[J]. Chinese Journal of Organic Chemistry, 2019 , 39(7) : 1983 -1989 . DOI: 10.6023/cjoc201812036
[1] Khorasanizadeh, S. Cell 2004, 116, 259.
[2] Kouzarides, T. Cell 2007, 128, 693.
[3] Brownell, J. E.; Zhou, J.; Ranalli, T.; Kobayashi, R.; Edmondson, D. G.; Roth, S. Y.; Allis, C. D. Cell 1996, 84, 843.
[4] Saha, R. N.; Pahan, K. Cell Death Differ. 2005, 13, 539.
[5] Zhang, J.; Zhong, Q. Cell. Mol. Life Sci. 2014, 71, 3885.
[6] Omar, K.; Thangue, N. B. L. Immunol. Cell Biol. 2012, 90, 85.
[7] Qiao, Z.; Ren, S.; Li, W.; Wang, X.; He, M.; Guo, Y.; Sun, L.; He, Y.; Ge, Y.; Yu, Q. Biochem. Biophys. Res. Commun. 2013, 434, 95.
[8] Ning, Z. Q.; Li, Z. B.; Newman, M. J.; Shan, S.; Wang, X. H.; Pan, D. S.; Zhang, J.; Dong, M.; Du, X.; Lu, X. P. Cancer Chemother. Pharmacol. 2012, 69, 901.
[9] Zhou, J.; Xie, H.; Liu, Z.; Luo, H. B.; Wu, R. J. Chem. Inf. Model. 2014, 54, 3162.
[10] Hu, X. S.; Wang, L.; Lin, L.; Han, X. H.; Dou, G. F.; Meng, Z. Y.; Shi, Y. K. Chin. J. Cancer Res. 2016, 28, 444.
[11] Jiao, J.; Hu, J. Q.; Fang, H. Chin. J. New Drug 2010, 386(in Chinese). (焦杰, 胡建强, 方浩, 中国新药杂志, 2010, 386.)
[12] Zhu, H. F.; Tong, H.; Gong, Y. Y.; Shao, S. Y.; Deng, C. M.; Yuan, W. Z.; Zhang, Y. M. J. Polym. Sci., Part A:Polym. Chem. 2012, 50, 2172.
[13] Zhao, W. N.; Ghosh, B.; Tyler, M.; Lalonde, J.; Joseph, N. F.; Kosaric, N.; Fass, D. M.; Tsai, L. H.; Mazitschek, R.; Haggarty, S. J. ACS Chem. Neurosci. 2018, 9, 2262.
[14] Yoon, H. J.; Yang, S. J.; Gong, Y. D. ACS Comb. Sci. 2017, 19, 738.
[15] Hornberger, K. R.; Badiang, J. G.; Salovich, J. M.; Kuntz, K. W.; Emmitte, K. A.; Cheung, M. Tetrahedron Lett. 2008, 49, 6348.
[16] Li, X. Y.; Inks, E. S.; Li, X. G; Hou, J. N.; Chou, C. J.; Zhang, J.; Jiang, Y. Q.; Zhang, Y. J.; Xu, W. F. J. Med. Chem. 2014, 57, 3324.
[17] Yu, C. G.; He, F.; Qu, Y.; Zhang, Q. Q.; Lv, J. H.; Zhang, X. N.; Xu, A. N.; Miao, P. N.; Wu, J. D. Bioorg. Med. Chem. 2018, 26, 1859.
/
| 〈 |
|
〉 |