

Chinese Journal of Organic Chemistry >
One-Pot, Two-Step Reductive Amination of Boronate Ester Containing Aromatic Amines and Aldehydes Using B2pin2 as Reductant
Received date: 2019-02-13
Revised date: 2019-03-15
Online published: 2019-03-29
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21172200, 21702191).
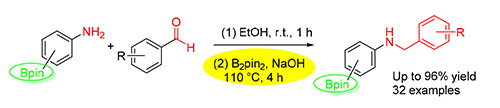
The aromatic amine functionality occupies a very important role in organic chemistry due to its prominence in biological and naturally occurring molecules. In addition, the synthesized secondary aromatic amines with pendant boronate ester are versatile intermediates in several organic transformations. The one-pot, two-step reductive amination of boron-containing primary aromatic amines and aldehydes has been achieved in the presence of NaOH in ethanol using B2pin2 as reductant. After extensive screening of various reaction parameters, such as base, reaction temperature, solvent, reaction time and protective gas, a series of secondary aromatic amines with pendant boronate ester and various functional groups were obtained in moderate to good yields under the optimal reaction conditions. This system features generally high yields and broad functional group tolerance. The boronate ester substituent is a very good handle to be further functionalized.
Liu Xueying , Liu Zhenwei , Guo Yuanyuan , Li Jingya , Zou Dapeng , Wu Yusheng , Wu Yangjie . One-Pot, Two-Step Reductive Amination of Boronate Ester Containing Aromatic Amines and Aldehydes Using B2pin2 as Reductant[J]. Chinese Journal of Organic Chemistry, 2019 , 39(7) : 2001 -2008 . DOI: 10.6023/cjoc201902011
[1] Pham, P. D.; Bertus, P.; Legoupy, S. Chem. Commun. 2009, 6207.
[2] For literature pertaining to the origins and definition of reductive amination, see:(a) Emerson, W. S. Org. React. 1948, 4, 174.
(b) Moore, M. L. Org. React. 1949, 5, 301.
(c) Nugenta, T. C.; El-Shazly, M. Adv. Synth. Catal. 2010, 352, 753.
[3] Chi, Y.; Zhou, Y.; Zhang, X. J. Org. Chem. 2003, 68, 4120.
[4] (a) Skucas, E.; Kong, J.; Krische, M. J. J. Am. Chem. Soc. 2007, 129, 7242.
(b) Park, J. W.; Chung, Y. K. ACS Catal. 2015, 5, 4846.
[5] Pagnoux-Ozherelyeva, A.; Pannetier, N.; Mbaye, M. D.; Gaillard, S.; Renaud, J.-L. Angew. Chem., Int. Ed. 2012, 51, 4976.
[6] Nasrollahzadeh, M. New J. Chem. 2014, 38, 5544.
[7] Huang, L.; Wang, Z.; Geng, L.; Chen, R.; Xing, W.; Wang, Y.; Huang, J. RSC Adv. 2015, 5, 56936.
[8] Gao, G.; Sun, P.; Li, Y.; Wang, F.; Zhao, Z.; Qin, Y.; Li, F. ACS Catal. 2017, 7, 4927.
[9] Abdel-Magid, A. F.; Carson, K. G.; Harris, B. D.; Maryanoff, C. A.; Shah, R. D. J. Org. Chem. 1996, 61, 3849.
[10] Roe, A.; Montgomery, J. A. J. Am. Chem. Soc. 1953, 75, 910.
[11] Tripathi, R. P.; Verma, S. S.; Pandey, J.; Tiwari, V. K. Curr. Org. Chem. 2008, 12, 1093.
[12] (a) Borch, R. F.; Bernstein, M. D.; Durst, H. D. J. Am. Chem. Soc. 1971, 93, 2897.
(b) Borch, R. F.; Hassid, A. I. J. Org. Chem. 1972, 37, 1673.
(c) Marchini, P.; Liso, G.; Reho, A.; Liberatone, F.; Moracci, F. M. J. Org. Chem. 1975, 40, 3453.
(d) Lane, C. F. Synthesis 1975, 135.
[13] (a) Abdel-Magid, A. F.; Maryanoff, C. A.; Carson, K. G. Tetrahedron Lett. 1990, 31, 5595.
(b) Kim, H. O.; Carrol, B.; Lee, M. S. Synth. Commun. 1997, 27, 2505.
(c) Tarasevich, V. A.; Kozlov, N. G. Russ. Chem. Rev. 1999, 68, 55.
[14] Borch, R. F.; Durst, H. D. J. Am. Chem. Soc. 1969, 91, 3996.
[15] (a) Ros, A.; Fernandez, R.; Lassaletta, J. M. Chem. Soc. Rev. 2014, 43, 3229.
(b) Hartwig, J. F. Acc. Chem. Res. 2012, 45, 864.
(c) Hartwig, J. F. Chem. Soc. Rev. 2011, 40, 1992.
(d) Mkhalid, I. A. I.; Barnard, J. H.; Marder, T. B.; Murphy, J. M.; Hartwig, J. F. Chem. Rev. 2010, 110, 890.
[16] (a) Xu, H.; Zhao, C.; Qian, Q.; Deng, W.; Gong, H. Chem. Sci. 2013, 4, 4022.
(b) Yu, X.; Wang, S.; Xu, H.; Gong, H. Org. Lett. 2011, 13, 2138.
(c) Liang, Z.; Xue, W.; Lin, K.; Gong, H. Org. Lett. 2014, 16, 5620.
(d) Zhang, G.; Xie, Y.; Wang, Z.; Liu, Y.; Huang, H. Chem. Commun. 2015, 51, 1850.
(e) Ke, M.; Song, Q. J. Org. Chem. 2016, 81, 3654.
(f) Doi, R.; Ohashi, M.; Ogoshi, S. Angew. Chem., Int. Ed. 2016, 55, 341.
(g) Ke, M.; Song, Q. Chem. Commun. 2017, 53, 2222.
(h) Chen, Z.; Wang, X. Org. Biomol. Chem. 2017, 15, 5790.
(i) Ke, M.; Song, Q. Adv. Synth. Catal. 2017, 359, 384.
(j) Lu, X.; Wang, Y.; Zhang, B.; Pi, J.; Wang, X.; Gong, T.; Xiao, B.; Fu, Y. J. Am. Chem. Soc. 2017, 139, 12632.
(k) Kuang, Z.; Li, B.; Song, Q. Chem. Commun. 2018, 54, 34.
[17] (a) Ojha, D. P.; Gadde, K.; Prabhu, K. R. Org. Lett. 2016, 18, 5062.
(b) Ding, W.; Song, Q. Org. Chem. Front. 2016, 3, 14.
(c) Wang, Q.; Yang, J.; Fang, D.; Ren, J.; Dong, B.; Zhou, B.; Zeng, B. Tetrahedron Lett. 2016, 57, 2587.
[18] (a) Laitar, D. S.; Müller, P.; Sadighi, J. P. J. Am. Chem. Soc. 2005, 127, 17196.
(b) Bae, S.; Lakshman, M. K. J. Org. Chem. 2008, 73, 1311.
(c) Kokatla, H. P.; Thomson, P. F.; Bae, S.; Doddi, V. R.; Lakshman, M. K. J. Org. Chem. 2011, 76, 7842.
(d) Xuan, Q.; Zhao, C.; Song, Q. Org. Biomol. Chem. 2017, 15, 5140.
[19] (a) Lu, H.; Geng, Z.; Li, J.; Zou, D.; Wu, Y. S.; W, Y. J. Org. Lett. 2016, 18, 2774.
(b) Yang, K.; Zhou, F.; Kuang, Z.; Gao, G.; Driver, T. Org. Lett. 2016, 18, 4088.
[20] Enthaler, S. Catal. Lett. 2012, 142, 1306.
[21] Xuan, Q.; Song Q. Org. Lett. 2016, 18, 4250.
[22] (a) Lu, H.; Wang, S.; Li, J.; Zou, D.; Wu, Y. S.; Wu, Y. J. Tetrahedron Lett. 2017, 58, 839.
(b) Zhi, W.; Li, J.; Zou, D.; Wu, Y.; Wu, Y. J. Org. Chem. 2017, 82, 12286.
(c) Ren, X.; Han, S.; Gao, X.; Li, J.; Zou, D.; Wu, Y.; Wu, Y. Tetrahedron Lett. 2018, 59, 1065.
(d) Zhi, W.; Li, J.; Zou, D.; Wu, Y.; Wu, Y. Tetrahedron Lett. 2018, 59, 537.
(e) Zhi, W.; Li, J.; Zou, D.; Wu, Y.; Wu, Y. Tetrahedron Lett. 2018, 59, 2736.
[23] (a) Geng, Z.; Zhang, Y.; Zheng, L.; Li, J.; Zou, D.; Wu, Y. J.; Wu, Y. S. Tetrahedron Lett. 2016, 57, 3063.
(b) Zhang, Y.; Geng, Z.; Li, J.; Zou, D.; Wu, Y.; Wu, Y. Adv. Synth. Catal. 2017, 359, 390.
(c) Zhu, M.; Qiu, Z.; Zhang, Y.; Du, H.; Li, J.; Zou, D.; Wu, Y.; Wu, Y. Tetrahedron Lett. 2017, 58, 2255.
(d) Zhu, M.; Du, H.; Li, J.; Zou, D.; Wu, Y.; Wu, Y. Tetrahedron Lett. 2018, 59, 1352.
[24] (a) Menet, C. J. M.; Blanc, J.; Hodges, A. J.; Burli, R. W.; Breccia, P.; Blackaby, W. P.; Van Rompaey, L. J. C.; Fletcher, S. R. WO 2010/010184, 2010.
(b) Liu, B.; Huang, J.; Zheng, C.; Zhang, Y.; Ouyang, L.; Mao, H.; Nie, B.; Xu, J.; Chen, H. CN 2015/10402418, 2015.
[25] Chen, Y.; Liu, S.; Cui, P.; Zhang, J.; Liu, Q.; Zhou, H. Tetrahedron Lett. 2019, 60, 327.
[26] Christopher, M. V.; Liliya, G. N.; David, W. N.; Heather, A. S.; Andreas, D.; Mark, O. B.; Felix, J. B.; Stephen, A. W. Can. J. Chem. 2001, 79, 1115.
[27] Wheaton, S. L.; Humanayun Kabir, S. M.; Zhang, H.; Vogels1, C. M.; Decken, A.; Westcott, S. Cent. Eur. J. Chem. 2010, 8, 725.
/
| 〈 |
|
〉 |