

Chinese Journal of Organic Chemistry >
Synthesis, Biological Activity and Molecular Docking of 4-Amino-5-substituted-1,2,4-triazole-3-thione Schiff Base
Received date: 2018-11-11
Revised date: 2019-01-21
Online published: 2019-04-08
Supported by
Project supported by the Provincial Key-point Natural Science Foundation of Shaanxi Province (No. 2016JZ003), the Innovation and Creativity Funds for Graduates in Northwest University (No. YZZ17137), the Innovation and Entrepreneurship Training Program for Undergraduates in Northwest University (No. 2018377).
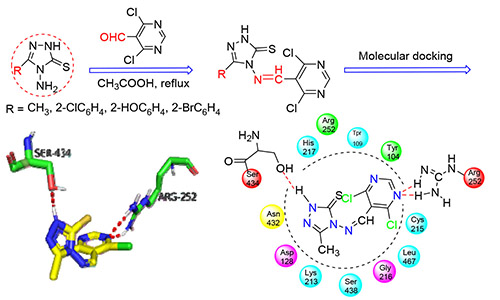
In this paper, the intermediate of 4-amino-5-methyl-1,2,4-triazole-3-thione (M1) was synthesized with thioformyl hydrazide and acetic acid, and 4-amino-5-aryl-1,2,4-triazole-3-thione (M2) was got by esterification, hydrazide, salt formation and cyclization with substituted benzoic acid. Four compounds of 1,2,4-triazole Schiff bases containing the pyrimidine ring M1-1, M2-1, M2-2 and M2-3 were obtained with 4,6-dichloro-5-pyrimidinecarboxaldehyde and M1/M2 according to the addition-elimination reaction. The structures of the compounds were characterized by elemental analysis, IR and 1H NMR spectra. The bioactivities and antibacterial mechanism of the title compounds were also studied by mycelial growth rate method and molecular docking. The results showed that the compounds had certain inhibitory effects on different fungi. Based on the values of EC50, compounds M1-1, M2-2 and M2-3 have better antifungal effects against the wheat gibberella than that of the standard drug (fluconazole), which is corresponding to the result of molecular docking.
Wu Shaojie , Lu Yiming , Lei Zhuonan , Jiang Yan , Zhang Wenhui , Qi Le , Ma Haixia , Ren Yinghui . Synthesis, Biological Activity and Molecular Docking of 4-Amino-5-substituted-1,2,4-triazole-3-thione Schiff Base[J]. Chinese Journal of Organic Chemistry, 2019 , 39(7) : 1939 -1944 . DOI: 10.6023/cjoc201811016
[1] Jin, R. Y. Ph. D. Dissertation, Northwest University, Xi'an, 2015 (in Chinese). (靳如意, 博士论文, 西北大学, 西安, 2015).
[2] Abele, E.; Abele, R.; Lukevics, E. Chem. Heterocycl. Compd. 2008, 44, 769.
[3] Kauthale, S.; Tekale, S.; Damale, M.; Sangshetti, J.; Pawar, R. Bioorg. Med. Chem. Lett. 2017, 27, 3891.
[4] Jiang, S. L.; Han, L. Chin. J. Org. Chem. 2012, 32, 930(in Chinese). (蒋绍亮, 韩亮, 有机化学, 2012, 32, 930.)
[5] Ren, G, Y. Ph. D. Dissertation, Northwest University, Xi'an, 2018 (in Chinese). (任国瑜, 博士论文, 西北大学, 西安, 2018.)
[6] Jin, R. Y.; Zeng, C. Y.; Liang, X. H.; Sun, X. H.; Liu, Y. F.; Wang, Y. Y.; Zhou, S. Bioorg. Chem. 2018, 80, 252.
[7] Sun, X. H.; Tao, Y.; Liu, Y. F.; Jia, Y.Q.; Chen, B. Acta Chim. Sinica 2008, 33, 234(in Chinese). (孙晓红, 陶燕, 刘源发, 贾婴琦, 陈邦, 化学学报, 2008, 33, 234.)
[8] Roman, G. Eur. J. Med. Chem. 2015, 89, 744.
[9] Ünver, Y.; Dügdü, E.; Sancak, K.; Mustafa, E. R.; Karaoglu, S. A. Turk. J. Chem. 2009, 33, 135.
[10] Tehrani, K., Mashayekhi, V.; Azerang, P.; Minaei, S.; Sardari, S.; Kobarfard, F. Ir. J. Pharm. Res. 2015, 14, 63.
[11] Tao, Y.; Sun, X. H.; Liu, Y. F.; Chen, B. Chem. Bioeng. 2016, 33, 50(in Chinese). (陶燕, 孙晓红, 刘源发, 陈邦, 化学与生物工程, 2016, 33, 50.)
[12] Jin, R. Y.; Sun, X. H.; Liu, Y. F.; Ma, H. X. Chemistry 2013, 76, 850(in Chinese). (靳如意, 孙晓红, 刘源发, 马海霞, 化学通报, 2013, 76, 850.)
[13] Zhao, P. L.; Chen, P.; Li, Q.; Hu, M. J.; Diao, P. C.; Pan, E. S.; You, W. W. Bioorg. Med. Chem. Lett. 2016, 26, 3680.
[14] Du, H. T.; Du, H. J. Chin. J. Org. Chem. 2010, 30, 138(in Chinese). (杜海堂, 杜海军, 有机化学, 2010, 30, 138.)
[15] Sahoo, K. P.; Sharma, R.; Pattanayak, P. Med. Chem. Res. 2010, 19, 129.
[16] Bhasin, G.; Srivastava, R.; Singh, R. Org. Prep. Proced. Int. 2017, 49, 371.
[17] Xie, W. L.; Zhang, J. G.; Ma, X. J.; Yang, W. Q.; Zhou, Y.; Tang, X. F.; Zou, Y.; Li, H.; He, J. J.; Xie, S. M.; Zhao, Y. H.; Liu, F. P. Chem. Biol. Drug. Des. 2015; 86, 1088.
[18] Magdy, S.; Omima, M. I. A.; Abdelrhman, E. M.; EI-Shetary, B. A. J. Mol. Struct. 2017, 1145, 330.
[19] Lu, W. T.; Sun, X. H.; Liu, Y. F.; Jin, R. Y. Chemistry 2012, 75, 362(in Chinese). (陆文婷, 孙晓红, 刘源发, 靳如意, 化学通报, 2012, 75, 362)
[20] Tang, G. H.; Zhang, Y.; Zhang Y. P.; Zhou, P. P.; Lin, Z. H.; Wang, Y. Q. Chem. J. Chin. Univ. 2017, 38, 2062(in Chinese). (唐光辉, 张娅, 张玉萍, 周朋朋, 林治华, 王远强, 高等化学学报, 2017, 38, 2062.)
[21] Beteringhe, A.; Racuciu, C.; Balan, C.; Stoican, E.; Patron, L. Adv. Mater. Res. 2013, 787, 236.
[22] El Bindary, A. A.; Shoair, A. F.; El-Sonbati, A. Z.; Diab, M. A.; Abdo, E. E. J. Mol. Liq. 2015, 212, 579.
[23] Wu, A. B. Ph.D. Dissertation, Huazhong Agricultural University, Wuhan, 2005 (in Chinese). (武爱波, 博士论文, 华中农业大学, 武汉, 2005.)
[24] Park, Y.; Cho, Y.; Lee, Y. H.; Lee, Y. W.; Rhee, S. J. Struct. Biol. 2016, 194, 395.
[25] Abd-Rabou, A. A.; Abdel-Wahab, B. F.; Bekheit, M. S. Chem. Pap. 2018, 72, 2231.
[26] El Ashry El Sayed H. El Ashry.; El Sayed H. El Tamany.; Mohy El Din Abd El Fattah.; Ahmed T. A. Boraei.; Heba M. Abd El-Nabi. Eu. J. Med. Chem. 2013, 66, 112.
[27] Zhou, J. H.; Li, J. J.; Li, J.; Ren, G. Y.; Ren, R. Y.; Ma, H. X. Chem. Res. Chin. Univ. 2017, 33, 866.
/
| 〈 |
|
〉 |