

Chinese Journal of Organic Chemistry >
One-Step Enol Esterification of 1,3-Dicarbonyls with Carboxylic Acids Activated by Perfluoroalkanosulfonyl Fluoride
Received date: 2019-01-10
Revised date: 2019-03-24
Online published: 2019-04-09
Supported by
Project supported by the National Natural Science Foundation of China (No. 21362022).
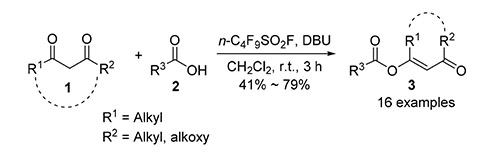
O-Acylation of 1,3-dicarbonyl compounds provides enol esters which act as precursors for the synthesis of chiral alcohols, natural products, heterocycles and functional materials. Perfluoroalkanosulfonyl fluoride (RfSO2F) is a class of excellent hydroxyl-activating reagent, and has been extensively developed and used in the formation of C-F, C-O, C-N and C-S bonds in organic synthesis. In this work one-step O-acylation of 1,3-dicarbonyl compounds (1,3-diketones and β-ketonic esters) with carboxylic acids activated by RfSO2F in alkaline media was disclosed, and the corresponding O-acylation products (enol esters) were generated in moderate to good yields. The optimized reaction conditions are as follows:1,8-diazabicyclo-[5.4.0]undec-7-ene (DBU) as base, CH2Cl2 as solvent, n-C4F9SO2F as activating reagent, room temperature for 30 min and the molar ratio of n(1,3-dicarbonyls):n(RCOOH):n(RfSO2F):n(DBU) being 1.0:1.0:1.0:4.0. A novel reagent for one-step O-acylation of 1,3-dicarbonyl compounds with carboxylic acids was developed. The application of RfSO2F in organic synthesis was further expanded.
Yan Zhaohua , Wang Yanmei , Jin Hong'ai , Ai Chengmei , Tian Weisheng . One-Step Enol Esterification of 1,3-Dicarbonyls with Carboxylic Acids Activated by Perfluoroalkanosulfonyl Fluoride[J]. Chinese Journal of Organic Chemistry, 2019 , 39(7) : 2042 -2047 . DOI: 10.6023/cjoc201901012
[1] Wang, Z.; Zhang, Z.; Liu, Y.; Zhang, W. Chin. J. Org. Chem. 2016, 36, 447(in Chinese). (王志惠, 张振锋, 刘燕刚, 张万斌, 有机化学, 2016, 36, 447.)
[2] Arakawa, H.; Aresta, M.; Armor, J. N.; Barteau, M. A.; Beckman, E. J.; Bell, A. T.; Bercaw, J. E.; Creutz, C.; Dinjus, E.; Gibson, D. H.; Goddard, W. A.; Goodman, D. W.; Keller, J.; Kubas, G. J.; Kung, H. H.; Lyons, J. E.; Manzer, L. E.; Marks, T. J.; Morokuma, K.; Nicholas, K. M.; Periana, R.; Que, L.; Rostrup-Nielson, J.; Sachtler, B. R.; Tumas, W. Chem. Rev. 2001, 101, 953.
[3] (a) Yoo, W.-J.; Li, C.-J. J. Org. Chem. 2006, 71, 6266.
(b). Rout, S. K.; Guin, S.; Banerjee, A.; Khatun, N.; Gogoi, A.; Patel, B. K. Org. Lett. 2013, 15, 4106.
(c) Dupuy, S.; Gasperini, D.; Nolan, S. P. ACS Catal. 2015, 5, 6918.
[4] Rottlander, M.; Knochel, P. J. Org. Chem. 1998, 63, 2038.
[5] Vorbruggen, H. Synthesis 2008, 1165.
[6] Yan, Z.; Wang, J.; Tian, W. Tetrahedron Lett. 2003, 44, 9383.
[7] Yan, Z.; Guan, C.; Yu, Z.; Tian, W. Tetrahedron Lett. 2013, 54, 5788.
[8] Yan, Z.; Tian, W.; Zeng, F.; Dai, Y. Tetrahedron Lett. 2009, 50, 2727.
[9] Yan, Z.-H.; Tian, H.; Zhao, D.-D.; Jin, H.-A.; Tian, W.-S. Chin. Chem. Lett. 2016, 27, 96.
[10] (a) Gui, J.; Wang, Y.; Tian, H.; Gao, Y.; Tian, W. Tetrahedron Lett. 2014, 55, 4233.
(b) Yan, Z.; Xu, Y.; Tian, W. Tetrahedron Lett. 2014, 55, 7186.
[11] Wang, W.; Jin, H.; Yan, Z.; He, M. Lin, S.; Tian, W. Tetrahedron Lett. 2017, 58, 3489.
[12] Yan, Z.; Jin, H.; Yu, X.; Wang, W.; Tian, W. Chin. J. Org. Chem. 2017, 37, 196(in Chinese). (严兆华, 金红爱, 余信权, 王汪阳, 田伟生, 有机化学, 2017, 37, 196.)
[13] Shimoyama, I.; Zhang, Y. T.; Wu, G. Z.; Negishi, E. Tetrahedron Lett. 1990, 31, 2841.
[14] Vellalath, S.; Van, K. N.; Romo, D. Tetrahedron Lett. 2015, 56, 3647.
[15] Tamura, Y.; Wada, A.; Okuyama, S.; Fukumori, S.; Hayashi, Y.; Gohda, N.; Kita, Y. Chem. Pharm. Bull. 1981, 29, 1312.
/
| 〈 |
|
〉 |