

Chinese Journal of Organic Chemistry >
A New Diarylethene Probe for Colorimetric Detection of CN- and Fluorescent Recognition of Zn2+/H2PO4-
Received date: 2019-01-24
Revised date: 2019-03-22
Online published: 2019-04-09
Supported by
Project supported by the National Natural Science Foundation of China (No. 41867053), and the Natural Science Foundation of Jiangxi Province (No. 20171ACB20025), the "5511" Science and Technology Innovation Talent Project of Jiangxi Province (No. 20165BCB18015), and the Project of the Science Funds of Jiangxi Education Office (No. GJJ180614).
A new diarylethene 1-(3,5-dimethylisoxazole-4-yl)-2-(2-methyl-5-[(3-aminonaphthol-2-yl)phenol-yl]-thiophene-3-yl)perfluorocyclopentene (1O) dual-response chemosensor has been synthesized, and its photochromic and fluorescent switch behaviors were systematically investigated by stimulation of lights and ions. The results indicated that 1O could serve as a CN-/F- "naked-eyes" colorimetric sensor with the color change from colorless to yellow, and act as a "Turn-on" fluorescence probe for specific detecting Zn2+. Moreover, the limits of detection of CN- and Zn2+ were determined to be 1.03×10-6 and 2.98×10-8 mol•L-1, respectively.
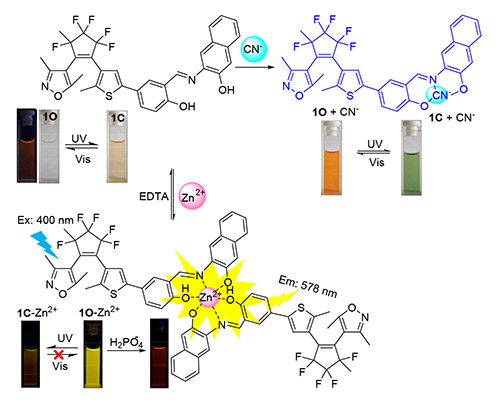
Key words: diarylethene; chemosensor; Zn2+; CN-; fluorescence enhancement
Diao Lu , Wang Renjie , Wang Niansheng , Liu Gang , Pu Shouzhi . A New Diarylethene Probe for Colorimetric Detection of CN- and Fluorescent Recognition of Zn2+/H2PO4-[J]. Chinese Journal of Organic Chemistry, 2019 , 39(7) : 1930 -1938 . DOI: 10.6023/cjoc201901038
[1] (a) Williams, R. J. P. The Biological Chemistry of the Elements, Clarendon, Oxford, 1991, p. 174.
(b) Bianchi, A.; Bowman-James, K.; García-España, E. Supramolecular Chemistry of Anions, Wiley-VCH, New York, 1997.
(c) Vazquez, M.; Fabrizzi, L.; Taglietti, A.; Pedrido, R. M.; Gonzalez-Noya, A. M.; Bermejo, M. R. Angew. Chem., Int. Ed. 2004, 43, 1962.
[2] (a) Silva, J.-J R. F. D.; Williams, R. J. P. The Biological Chemistry of the Elements:the Inorganic Chemistry of Life, Oxford University Press, Oxford, 2001, p. 315.
(b) Vallee, B. L.; Auld, D. S. Acc. Chem. Res. 1993, 26, 543.
(c) Bush, I.; Pettingell, W. H.; Multhaup, G.; Paradis, M. D.; Vonsattel, J. P.; Gusella, J. F.; Beyreuther, K.; Masters, C. L.; Tanzi, R. E. Science 1994, 265, 1464.
(d) Fraker, P. J.; King, L. E. Annu. Rev. Nutr. 2004, 24, 277.
(e) Cuajungco, M. P.; Lees, G. J. Neurobiol. Dis. 1997, 4, 137.
(f) Frassinetti, S.; Bronzetti, G. L.; Caltavuturo, L.; Cini, M. Della Croce, C. J. Environ. Pathol. Toxicol. Oncol. 2006, 25, 597.
[3] (a) Lai, J.; Moxey, A.; Nowak, G.; Vashum, K.; Bailey, K.; McEvoy, M. J. Affective Disord. 2012, 136, 31.
(b) Weiss, J. H.; Sensi, S. L.; Koh, J. Y. Trends Pharmacol. Sci. 2000, 21, 395.
(c) Maes, M.; Vos, N. D.; Demedts, P.; Wauters, A.; Neels, H. J. Affective Disord. 1999, 56, 189.
[4] Carol, P.; Sreejith, S.; Ajayaghosh, A. Chem. Asian. J. 2007, 2, 338.
[5] (a) Zhang, J. F.; Bhuniya, S. Y.; Lee, H.; Bae, C.; Lee, J. H.; Kim, J. S. Tetrahedron Lett. 2010, 51, 3719.
(b) Voegelin, A.; Pfister, S.; Scheinost, A. C.; Marcus, M. A.; Kretzschmar, R. Environ. Sci. Technol. 2005, 39, 6616.
(c) Callender, E.; Rice, K. C. Environ. Sci. Technol. 2000, 34, 232.
(d) Li, L.; Dang, Y. Q.; Li, H. W.; Wang, B.; Wu, Y. Tetrahedron Lett. 2010, 51, 618.
[6] (a) Tang, L.; Zhou, P.; Zhong, K.; Hou, S. Sens. Actuators, B:2013, 182, 439.
(b) Kumari, N.; Jha, S.; Bhattacharya, S. J. Org. Chem. 2011, 76, 8215.
(c) Zhang, W.; Xu, K.; Yue, L.; Shao, Z.; Feng, Y.; Fang, M. Dyes Pigm. 2017, 137, 560.
(d) Shan, Y.; Wu, Q.; Sun, N.; Sun, Y.; Cao, D.; Liu, Z. Macromol. Chem. Phys. 2017, 186, 295.
(e) Liu, T.; Huo, F.; Li, J.; Cheng, F.; Ying, C. Sens. Actuators, B 2017, 239, 526.
[7] (a) Hachiya, H.; Ito, S.; Fushinuki, Y.; Masadome, T.; Asano, Y.; Imato, T. Talanta 1999, 48, 997.
(b) Wang, L. Y.; Li, L. Q.; Cao, D. R. Sens. Actuator, B 2017, 239, 1307.
(c) Kalpana, P.; Suganya, S.; Velmathi, S. Spectrochim. Acta, A 2017, 171, 162.
(d) Li, H.; Zhao, P.; Zou, N.; Wang, H.; Sun, K. Tetrahedron Lett. 2017, 58, 30.
[8] Saenger, W. Principles of Nucleic Acid Structure, Springer, New York, 1988.
[9] (a) Paredes, J. M.; Giron, M. D.; Ruedas-Rama, M. J.; Orte, A.; Crovetto, L.; Talavera, E. M.; Salto, R.; Alvarez-Pez, J. M. J. Phys. Chem. B 2013, 117, 8143.
(b) Bhaumik, C.; Das, S.; Maity, D.; Baitalik, S. Dalton Trans. 2011, 40, 11795.
[10] (a) Irie, M. Chem. Rev. 2000, 100, 1685.
(b) Tian, H.; Yang, S. Chem. Soc. Rev. 2004, 35, 85.
(c) Irie, M.; Fukaminato, T.; Sasaki, T. Nature 2002, 420, 759.
(d) Fu, Y. L.; Fan, C. B.; Liu, G.; Pu, S. Z. Sens. Actuators, B 2017, 239, 295.
(e) Zhang, X.-X.; Wang, R. J.; Fan, C. B.; Liu, G.; Pu, S. Z. Dyes Pigm. 2017, 139, 208.
[11] (a) Wang, R. J.; Wang, N. S.; Pu, S. Z.; Zhang, X.-X.; Liu, G.; Dai, Y. F. Dyes Pigm. 2017, 146, 445.
(b) Wang, R. J.; Wang, N. S.; Tu, Y.-Y.; Liu, G.; Pu, S. Z. J. Photochem. Photobiol. A 2018, 364, 32.
(c) Wang, R. J.; Diao, L.; Ren, Q. W.; Liu, G.; Pu, S. Z. ACS Omega 2019, 4, 309.
[12] Irie, M.; Fukaminato, T.; Matsuda, K.; Kobatake, S. Chem. Rev. 2014, 114, 12174.
[13] Wu, J. S.; Liu, W. M.; Zhuang, X. Q. Org. Lett. 2007, 9, 33.
[14] Wang, H.; Wang, B.; Shi, Z. Biosens. Bioelectron. 2015, 65, 91.
[15] (a) Dong, W. K.; Li, X. L.; Wang, L.; Zhang, Y.; Ding, Y. J. Sens. Actuators, B 2016, 229, 370.
(b) Feng, E. T.; Tu, Y.-Y.; Fan, C. B.; Liu, G.; Pu, S. Z. RSC Adv. 2017, 7, 50188.
[16] Yao, K.; Fu, J. X.; Chang, Y. X.; Li, B.; Yang, L.; Xu, K. X. Spectrochim. Acta, Part A 2018, 205, 410.
[17] Sabyasachi, T.; Sudipta, D.; Milan, G.; Mahuya, B.; Kumar, H. S.; Pratim, M. P.; Debasis, D. Spectrochim. Acta, Part A 2019, 209, 170.
[18] Purkait, R.; Chattopadhyay, D. A.; Sinha, C. Spectrochim. Acta, Part A 2019, 207, 164.
[19] Karar, M.; Paul, S.; Biswas, B. Dalton. Trans. 2018, 276, 560.
[20] Cui, S. Q.; Pu, S. Z.; Liu, G. Spectrochim. Acta, Part A 2014, 132, 339.
/
| 〈 |
|
〉 |