

Chinese Journal of Organic Chemistry >
Regioselective Ring-Opening Reaction of Cyclopropene Carboxylate Promoted by N-Bromosuccinimide
Received date: 2018-12-16
Revised date: 2019-03-28
Online published: 2019-04-11
Supported by
Project supported by the Zhejiang Provincial Natural Science Foundation of China (Nos. LY18B020018, LY15B020004) and the National Science Foundation of China (No. 21602197).
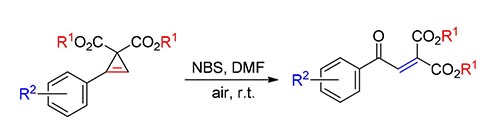
The cyclopropene compound contains an intra carbon-carbon double bond structure, which leads to distinctive active chemical reactivity due to the large ring tension. In this paper, the N-bromosuccinimide-promoted regioselective ring-opening of cyclopropene dicarboxylates to give functionalized α,β-unsaturated carboxylic acid ester compounds was studied. The reaction conditions are mild and the operation is simple.
Xu Lulu , Ye Qianwen , Cheng Dongping , Li Xiaonian , Xu Xiaoliang . Regioselective Ring-Opening Reaction of Cyclopropene Carboxylate Promoted by N-Bromosuccinimide[J]. Chinese Journal of Organic Chemistry, 2019 , 39(9) : 2645 -2649 . DOI: 10.6023/cjoc201812029
[1] (a) Wang, Y.; Fordyce, E. A. F.; Chen, F. Y.; Lam, H. Y. Angew. Chem. Int. Ed. 2008, 47, 7350.
(b) Goto, T.; Takeda, K.; Shimada, N.; Nambu, H.; Anada, M.; Shiro, M.; Ando, K.; Hashimoto, S. Angew. Chem., Int. Ed. 2011, 50, 6803.
(c) Chen, J.; Ma, S. Chem. Asian J. 2010, 5, 2415.
(d) Song, C.; Ju, L.; Wang, M.; Liu, P.; Zhang, Y.; Wang, J.; Xu, Z. Chem. Eur. J. 2013, 19, 3584.
(e) Rubin, M.; Rubina, M.; Gevorgyan, V. Chem. Rev. 2007, 107, 3117.
(f) Archambeau, A.; Miege, F.; Meyer, C.; Cossy, J. Acc. Chem. Res. 2015, 48, 1021.
[2] (a) Chuprakov, S.; Rubin, M.; Gevorgyan, V. J. Am. Chem. Soc. 2005, 127, 3714.
(b) Paswa, A. Acc. Chem. Res. 1979, 12, 310.
(c) Chen, J.; Ni, S.; Ma, S. Synlett 2011, 931.
(d) DeMartino, M. P.; Chen, K.; Baran, P. S. J. Am. Chem. Soc. 2008, 130, 11546.
(e) Zhu, P. L.; Tang, X. Y.; Shi, M. Chem. Commun. 2016, 52, 7245.
(f) Song, C.; Sun, D.; Peng, X.; Bai, J.; Zhang, R.; Hou, S.; Wang, J.; Xu, Z. Chem. Commun. 2013, 49, 9167.
(g) Padwa, A. Acc. Chem. Res. 1979, 12, 310.
[3] (a) Welch, J. G.; Magid, R. M. J. Am. Chem. Soc. 1967, 89, 5300.
(b) Kubota, K.; Mori, S.; Nakamura, E. J. Am. Chem. Soc. 1998, 120, 13334.
(c) Liao, L.; Fox, J. M. J. Am. Chem. Soc. 2002, 124, 14322.
(d) Liao, L.; Zhang, F.; Yan, N.; Golen, J. A.; Fox, J. M. Tetrahedron. 2004, 60, 1803.
(e) Miege, F.; Meyer, J.; Cossy, J. Org. Lett. 2010, 12, 4144.
[4] (a) Ma, S.; Zhang, J.; Cai, Y.; Lu, L. J. Am. Chem. Soc. 2003, 125, 13954.
(b) Liu, Y.; Ma, S. Org. Lett. 2012, 14, 720.
(c) Liu, Y.; Yu, Q.; Ma, S. Eur. J. Org. Chem. 2013, 15, 3033.
(d) Wang, Y.; Lam, H. W. J. Org. Chem. 2009, 74, 1353.
(e) Wang, Y.; Fordyce, E. A. F.; Chen, F. Y.; Lam, H. W. Angew. Chem. Int. Ed. 2008, 47, 7350.
[5] (a) Fordyce, E. A. F.; Wang, Y.; Luebbers, T.; Lam, H. W. Chem. Commun. 2008, 1124.
(b) Maksic, M. E.; Golic, M.; Tolic, L. P. J. Org. Chem. 1995, 489, 35.
(c) Liao, L.; Yan, N.; Fox, J. M. Org. Lett. 2004, 6, 4937.
[6] (a) Wang, H.; Zhang, L.; Tu, Y.; Xiang, R.; Guo, Y. L.; Zhang, J. L. Angew. Chem. Int. Ed. 2018, 57, 15787.
(b) Fulton, J. L.; Horwitz, M. A.; Bruske, E. L.; Johnson, J. S. J. Org. Chem. 2018, 83, 3385.
(c) Horwitz, M. A.; Fulton, J. L.; Johnson, J. S. Org. Lett. 2017, 19, 5783.
[7] Ye, Q. W.; Ye, H. Q.; Cheng, D. P.; Li, X. N.; Xu, X. L. Tetrahedron Lett. 2018, 59, 2546.
[8] (a) Ye, H. Q.; Ye, Q. W.; Cheng, D. P.; Li, X. N.; Xu, X. L. Tetrahedron Lett. 2018, 59, 2046.
(b) Dai, X. J.; Cheng, D. P.; Guan, B. C.; Mao, W. J.; Xu, X. L.; Li, X. N. J. Org. Chem. 2014, 79, 7212.
(c) Ye, Q. W.; Xu, X. L.; Cheng, D. P.; Guan, B. C.; Ye, H. F.; Li, X. N. ARKIVOC 2017, 314.
(d) Chen, J.; Cen, J.; Xu, X. L.; Li, X. N. Catal. Sci. Technol. 2016, 6, 349.
[9] (a) Liu, K.; Jiang, H. J.; Li, Na.; Li, H.; Wang, J.; Zhang, Z. Z.; Yu, J. J. Org. Chem. 2018, 83, 6815.
(b) Huang, P.; Peng, X.; Hu, D.; Liao, H.; Tang, S.; Liu, L. Org. Biomol. Chem. 2017, 15, 9622.
(c) Wang, H.; Huang, L.; Cao, X.; Liang, D.; Peng, A. Y. Org. Biomol. Chem. 2017, 15, 7396.
(d) Wang, D.; Yan, Z.; Xie, Q.; Zhang, R.; Lin, S.; Wang, Y. Org. Biomol. Chem. 2017, 15, 1998.
(e) Sathe, P. A.; Karpe, A. S,; Parab, A. A.; Parade, B. S.; Vadagaonkar, K. S.; Chaskar, A. C. Tetrahedron Lett. 2018, 59, 2820.
(f) Qian, M.; Qin, B.; Yuan, H. Y.; Li, W. L.; Zhang, J. P. J. Comput. Chem. 2018, 39, 2324.
(g) Pardeshi, S. D.; Sathe, P. A.; Wadagaonkar, K. S.; Chaskar, A. C. Adv. Synth. Catal. 2017, 359, 4217。
(h) Shinde, M. H.; Kshirsagar, U. A. Green Chem. 2016, 18, 1455.
[10] (a) Waser, J.; Gaspar, B.; Nambu, H.; Carreira, E. M. J. Am. Chem. Soc. 2006, 128, 11693.
(b) Kang, T.; Kim, Y.; Lee, D.; Wang, Z.; Chang, S. J. Am. Chem. Soc. 2014, 136, 4141.
(c) Chen, S. Y.; Feng, B. Y.; Zheng, X. S.; Yin, J. L.; Yang, S. P.; You, J. S. Org. Lett. 2017, 19, 2502.
(d) Keipour, H.; Ollevier, T. Org. Lett. 2017, 19, 5736.
[11] (a) Simone, F. D.; Saget, T.; Benfatti, F.; Almeida, S.; Waser, J. Chem. Eur. J. 2011, 17, 14527.
(b) Jiang, Y.; Khong, V. Z. Y.; Lourdusamy, E.; Park, C. M. Chem. Commun. 2012, 48, 3133.
(c) Pandit, R. P.; Kim, S. H.; Lee, Y. R. Adv. Synth. Catal. 2016, 358, 3586.
(d) Keipour, H.; Jalba, A.; Laurin, L. D.; Ollevier, T. J. Org. Chem. 2017, 82, 3000.
[12] (a) Bobes, F. G.; Fenster, M. D. B.; Kiau, S.; Kolla, L.; Ko-lotuchin, S.; Soumeillant, M. Adv. Synth. Catal. 2008, 350, 813.
(b) Dange, N. S.; Robert, F.; Landais, Y. Org. Lett. 2016, 18, 6156.
(c) Wheeler, T.; Ray, J. J. Org. Chem. 1987, 52, 4875.
/
| 〈 |
|
〉 |