

Chinese Journal of Organic Chemistry >
Cross-Linked Chitosan Bead Supported Copper Complex in Water as a Green and Efficient Catalytic Protocol for Ullmann Reaction
Received date: 2019-01-14
Revised date: 2019-03-25
Online published: 2019-04-11
Supported by
Project supported by the National Natural Science Foundation of China (No. 21476194), and the National Key Research and Development Program of China (No. 2016YFB0301800).
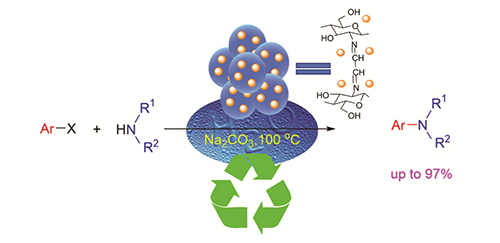
A green, efficient, and recyclable catalytic protocol for Ullmann C-N reaction in water was developed. The catalyst Chi-Gly@CuI was prepared by the cross-linking reaction of chitosan bead with glyoxal and subsequently anchored with copper salt. Chi-Gly@CuI bead of 0.3 mm in mean diameter possesses porous micro-structure demonstrated by scanning electron microscope (SEM). The structure of Chi-Gly@CuI was characterized by Fourier transform infrared spectroscopy (FTIR), thermogravimetric analysis (TG), X-ray diffraction (XRD), inductively coupled plasma-atomic emission spectrometry (ICP-AES), and X-ray photoelectron spectroscopy (XPS). This catalytic protocol for Ullmann reaction in water exhibited high applicability, from which the corresponding coupling products were afforded in good to excellent yields. Chi-Gly@CuI could be easily separated from products by simple filtration almost without weight loss. Most notably, after 10 times of recycling, its catalytic activity and chemical stability were still maintained.
Lü Xiaomei, Ruan Jiancheng, Chen Xinzhi, Qian Chao . Cross-Linked Chitosan Bead Supported Copper Complex in Water as a Green and Efficient Catalytic Protocol for Ullmann Reaction[J]. Chinese Journal of Organic Chemistry, 2019 , 39(6) : 1720 -1726 . DOI: 10.6023/cjoc201901018
[1] Ferlin, F.; Trombettoni, V.; Luciani, L.; Fusi, S.; Piermatti, O.; Santoro, S.; Vaccaro, L. Green Chem. 2018, 20, 1634.
[2] Bariwal, J.; Van der Eycken, E. Chem. Soc. Rev. 2013, 42, 9283.
[3] Monnier, F.; Taillefer, M. Angew. Chem., Int. Ed. 2009, 48, 6954.
[4] Hassan, J.; Sevignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Chem Rev 2002, 102, 1359.
[5] Beletskaya, I. P.; Cheprakov, A. V. Coord. Chem. Rev. 2004, 248, 2337.
[6] Sambiagio, C.; Marsden, S. P.; Blacker, A. J.; McGowan, P. C. Chem. Soc. Rev. 2014, 43, 3525.
[7] Monnier, F.; Taillefer, M., Angew. Chem., Int. Ed. 2008, 47, 3096.
[8] Hartwig, J. F. Angew. Chem., Int. Ed. 1998, 37, 2046.
[9] Klapars, A.; Antilla, J. C.; Huang, X. H.; Buchwald, S. L. J. Am. Chem. Soc. 2001, 123, 7727.
[10] Job, G. E.; Buchwald, L. Org. Lett. 2002, 4, 3703.
[11] Klapars, A.; Huang, X. H.; Buchwald, S. L. J. Am. Chem. Soc. 2002, 124, 7421.
[12] Ma, D. W.; Cai, Q. Synlett 2004, (1), 128.
[13] Cheng, D. P.; Gan, F. F.; Qian, W. X.; Bao, W. L. Green Chem. 2008, 10, 171.
[14] Kwong, F. Y.; Klapars, A.; Buchwald, S. L. Org. Lett. 2002, 4, 581.
[15] Kwong, F. Y.; Buchwald, S. L. Org. Lett. 2003, 5, 793.
[16] Antilla, J. C.; Baskin, J. M.; Barder, T. E.; Buchwald, S. L. J. Org. Chem. 2004, 69, 5578.
[17] Cristau, H. J.; Cellier, P. P.; Spindler, J. F.; Taillefer, M. Chem.-Eur. J. 2004, 10, 5607.
[18] Choudary, B. M.; Sridhar, C.; Kantam, M. L.; Venkanna, G. T.; Sreedhar, B. J. Am. Chem. Soc. 2005, 127, 9948.
[19] Strieter, E. R.; Blackmond, D. G.; Buchwald, S. L. J. Am. Chem. Soc. 2005, 127, 4120.
[20] Zhang, H.; Cai, Q.; Ma, D. W. J. Org. Chem. 2005, 70, 5164.
[21] Zhu, W.; Ma, D. W. J. Org. Chem. 2005, 70, 2696.
[22] Shafir, A.; Buchwald, S. L. J. Am. Chem. Soc. 2006, 128, 8742.
[23] Ma, D. W.; Cai, Q. A. Acc. Chem. Res. 2008, 41, 1450.
[24] Cassez, A.; Ponchel, A.; Hapiot, F.; Monflier, E. Org. Lett. 2006, 8, 4823.
[25] Xue, C. H.; Palaniappan, K.; Arumugam, G.; Hackney, S. A.; Liu, J.; Liu, H. Y. Catal. Lett. 2007, 116, 94.
[26] Ngah, W. S. W.; Fatinathan, S. Chem. Eng. J. 2008, 143, 62.
[27] Baig, R. B. N.; Varma, R. S. Green Chem. 2013, 15, 1839.
[28] Dekamin, M. G.; Azimoshan, M.; Ramezani, L. Green Chem. 2013, 15, 811.
[29] Zhang, G. F.; Luan, Y. X.; Han, X. W.; Wang, Y.; Wen, X.; Ding, C. R.; Gao, J. R. Green Chem. 2013, 15, 2081.
[30] Baig, R. B. N.; Nadagouda, M. N.; Varma, R. S. Green Chem. 2014, 16, 2122.
[31] Shen, C.; Xu, J.; Yu, W. B.; Zhang, P. F. Green Chem. 2014, 16, 3007.
[32] Yang, B.; Mao, Z. X.; Zhu, X. H.; Wan, Y. Q. Catal. Commun. 2015, 60, 92.
[33] Cui, Q.; Zhao, H.; Luo, G.; Xu, J. Ind. Eng. Chem. Res. 2017, 56, 143.
[34] Ma, L.; Su, Y.; Chen, J.; Xu, J. Ind. Eng. Chem. Res. 2017, 56, 12655.
[35] Su, Y.; Ma, L.; Chen, J.; Xu, J. Carbohydr. Polym. 2017, 175, 113.
[36] Tong, J. H.; Zhen, L.; Xia, C. G. J. Mol. Catal. A 2005, 231(1-2), 197.
[37] Anuradha; Kumari, S.; Pathak, D. D. Tetrahedron Lett. 2015, 56, 4135.
[38] Keshipour, S.; Mirmasoudi, S. S. Carbohydr. Polym. 2018, 196, 494.
[39] Gioia, C.; Ricci, A.; Bernardi, L.; Bourahla, K.; Tanchoux, N.; Robitzer, M.; Quignard, F. Eur. J. Org. Chem. 2013, (3), 588.
[40] Chanda, A.; Fokin, V. V. Chem. Rev. 2009, 109, 725.
[41] Krause, N. Curr. Opin. Green Sustainable Chem. 2017, 7, 18.
[42] La Sorella, G.; Strukul, G.; Scarso, A. Green Chem 2015, 17, 644.
[43] Lipshutz, B. H.; Ghorai, S.; Cortes-Clerget, M. Chemistry 2018, 24, 6672.
[44] Rani, D.; Singla, P.; Agarwal, J. Carbohydr. Polym. 2018, 202, 355.
[45] Ge, X.; Qian, C.; Chen, X. Z. Tetrahedron:Asymmetry 2014, 25, 1450.
[46] Ge, X.; Qian, C.; Chen, Y. B.; Chen, X. Z. Tetrahedron:Asymmetry 2014, 25, 596.
[47] Ge, X.; Chen, X. Z.; Qian, C.; Zhou, S. D. RSC Adv. 2016, 6, 58898.
[48] Ge, X.; Chen, X. Z.; Qian, C.; Zhou, S. D. RSC Adv. 2016, 6, 29638.
[49] Ge, X.; Sun, F.; Liu, X.; Chen, X.; Qian, C.; Zhou, S. New J. Chem. 2017, 41, 13175.
[50] Liu, X.; Chang, S.; Chen, X.; Ge, X.; Qian, C. New J. Chem. 2018, 42, 16013.
[51] Sowmya, A.; Meenakshi, S. Int. J. Biol. Macromol. 2014, 64, 224.
[52] Saha, S.; Das, S.; Sen, D.; Ghorai, U. K.; Mazumder, N.; Gupta, B. K.; Chattopadhyay, K. K. J. Mater. Chem. C 2015, 3, 6786.
[53] Kumar, M.; Bhatt, V.; Nayal, O. S.; Sharma, S.; Kumar, V.; Thakur, M. S.; Kumar, N.; Bal, R.; Singh, B.; Sharma, U. Catal. Sci. Technol. 2017, 7, 2857.
[54] Zeng, L. X.; Chen, Y. F.; Zhang, Q. Y.; Guo, X. M.; Peng, Y. N.; Xiao, H. J.; Chen, X. C.; Luo, J. W. Carbohydr. Polym. 2015, 130, 333.
[55] Zhou, Y. T.; Branford-White, C.; Nie, H. L.; Zhu, L. M. Colloid Surface B 2009, 74, 244.
/
| 〈 |
|
〉 |