

Chinese Journal of Organic Chemistry >
Visible-Light-Induced[3+2] Annulation of Cyclopropylamines with 1,2-Diketone Derivatives
Received date: 2019-02-21
Revised date: 2019-03-27
Online published: 2019-04-11
Supported by
Project supported by the National Natural Science Foundation of China (No. 21572184), the Natural Science Foundation of Fujian Province (No. 2017J06006), and the Fundamental Research Funds for the Central Universities (No. 20720160027).
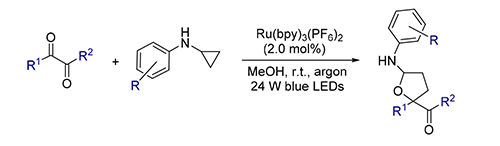
A visible-light-induced[3+2] annulation of arylcyclopropylamines and 1,2-diarylethanediones was report. A series of α-amino tetrahydrofuran derivatives were synthesized in moderate to good isolated yields under mild reaction conditions. This method would provide an efficient and convenient approach to α-amino tetrahydrofurans which are potentially important buiding blocks in bioactive compounds.
Key words: photoredox; cyclopropylamines; 1,2-diketone derivatives; [3+2] annulation
Dai Xuemei , Li Yanjun , Zhang Shaonan , Gong Lei . Visible-Light-Induced[3+2] Annulation of Cyclopropylamines with 1,2-Diketone Derivatives[J]. Chinese Journal of Organic Chemistry, 2019 , 39(6) : 1711 -1719 . DOI: 10.6023/cjoc201902022
[1] Cheng, X. H.; Hii, K. K. Tetrahedron 2001, 57, 5445.
[2] Kunz, K. R.; Iyengar, B. S.; Dorr, R. T.; Alberts, D. S.; Remers, W. A. J. Med. Chem. 1991, 34, 2281.
[3] Lockhoff, O.; Stadler, P. Carbohydr. Res. 1998, 314, 13.
[4] Takeuchi, Y.; Chang, M. R.; Hashigaki, K.; Yamato, M. Chem. Pharm. Bull. 1991, 39, 1629.
[5] (a) Eliel, E. L.; Daignault, R. A. J. Org. Chem. 1965, 30, 2450~2451.
(b) Glacet, C.; Veron, D. Bull. Soc. Chim. Fr. 1965, 1789.
[6] For selected reviews on visible-light photoredox catalysis, see:
(a) Narayanam, J. M. R.; Stephenson, C. R. J. Chem. Soc. Rev. 2011, 40, 102.
(b) Xuan, J.; Xiao, W. J. Angew. Chem., Int. Ed. 2012, 51, 6828.
(c) Shi, L.; Xia, W. Chem. Soc. Rev. 2012, 41, 7687.
(d) Xi, Y.; Yi, H.; Lei, A. Org. Biomol. Chem. 2013, 11, 2387.
(e) Prier, C. K.; Rankic, D. A.; MacMillan, D. W. C. Chem. Rev. 2013, 113, 5322.
(f) Dai, X. J.; Xu, X. L.; Li, X. N. Chin. J. Org. Chem. 2013, 33, 2406 (in Chinese).(戴小军, 许孝良, 李小年, 有机化学, 2013, 33, 2406.)
(g) Guan, B. C.; Xu, X. L.; Wang, H; Li, X. N. Chin. J. Org. Chem. 2016, 36, 1564 (in Chinese).(关保川, 许孝良, 王红, 李小年, 有机化学, 2016, 36, 1564.)
(h) Shaw, M. H.; Twilton, J.; MacMillan, D. W. C. J. Org. Chem. 2016, 81, 6898.
(i) Skubi, K. L.; Blum, T. R.; Yoon. T. P. Chem. Rev. 2016, 116, 10035.
(j) Nakajima, K.; Miyake, Y.; Nishibayashi, Y. Acc. Chem. Res. 2016, 49, 1946.
(k) Liu, W.; Zheng, X. Y.; Zeng, J. G.; Cheng, P. Chin. J. Org. Chem. 2017, 37, 1 (in Chinese).(刘薇, 郑昕宇, 曾建国, 程辟, 有机化学, 2017, 37, 1.)
(l) Ruan, L. H.; Dong, Z. C.; Chen, C. X.; Wu, S.; Sun, J. Chin. J. Org. Chem. 2017, 37, 2544 (in Chinese).(阮利衡, 董振诚, 陈春欣, 吴爽, 孙京, 有机化学, 2017, 37, 2544.)
(m) Dai, X. Q.; Zhu, Y. B.; Xu, X. L.; Weng, J. Q. Chin. J. Org. Chem. 2017, 37, 577 (in Chinese).(戴小强, 朱亚波, 许孝良, 翁建全, 有机化学, 2017, 37, 577.)
(n) Xu, W. X.; Dai, X. Q; Xu, H. J.; Weng, J. Q. Chin. J. Org. Chem. 2018, 38, 2807 (in Chinese).(徐雯秀, 戴小强, 徐涵靖, 翁建全, 有机化学, 2018, 38, 2807.)
(o) Bai, Q. F.; He, J. Y.; Zhu, X. Q.; Feng, G. F.; Jin, C. A. Chin. J. Org. Chem. 2019, 39, 527 (in Chinese).(白其凡, 何静耀, 祝小青, 冯高峰, 金城安, 有机化学, 2019, 39, 527.)
[7] For examples of visible-light photoredox catalysis, see:
(a) Nicewicz, D. A.; MacMillan, D. W. C. Science 2008, 322, 77.
(b) DiRocco, D. A.; Rovis, T. J. Am. Chem. Soc. 2012, 134, 8094.
(c) Rono, L. J.; Yayla, H. G.; Wang, D. Y.; Armstrong, M. F.; Knowles, R. R. J. Am. Chem. Soc. 2013, 135, 17735.
(d) Alonso, R.; Bach, T. Angew. Chem., Int. Ed. 2014, 53, 4368.
(e) Huo, H. H.; Shen, X. D.; Wang, C. Y.; Zhang, L. L.; Rose, P.; Chen, L. A.; Harms, K.; Marsch, M.; Hilt, G.; Meggers, E. Nature 2014, 515, 100.
(f) Espelt, L. R.; McPherson, I. S.; Wiensch, E. M. Yoon, T. P. J. Am. Chem. Soc. 2015, 137, 2452.
(g) Ding, W.; Lu, L. Q.; Zhou, Q. Q.; Wei, Y.; Chen, J. R.; Xiao, W. J. J. Am. Chem. Soc. 2017, 139, 63.
(h) Ding, W.; Lu, L. Q.; Zhou, Q. Q.; Wei, Y.; Chen, J. R.; Xiao, W. J. J. Am. Chem. Soc. 2017, 139, 63.
(i) Zhou, Q. Q.; Liu, D.; Xiao, W. J.; Lu, L. Q. Acta Chim. Sinica 2017, 75, 110 (in Chinese).(周泉泉, 刘丹, 肖文精, 陆良秋, 化学学报, 2017, 75, 110.)
(j) Wu, Z. J.; Wang, J. Acta Chim. Sinica 2017, 75, 74 (in Chinese).(吴自俊, 汪舰, 化学学报, 2017, 75, 74.)
(k) Wu, J.; Li, J. W.; Li, H.; Zhu, C. Y. Chin. J. Org. Chem. 2017, 37, 2203 (in Chinese).(吴江, 李嘉雯, 李昊, 朱纯银, 有机化学, 2017, 37, 2203.)
(l) Ye, H; Xiao, C; Lu, L. Q. Chin. J. Org. Chem. 2018, 38, 1897 (in Chinese).(叶辉, 肖聪, 陆良秋, 有机化学, 2018, 38, 1897.)
[8] (a) Kohls, P.; Jadhav, D.; Pandey, G.; Reiser, O. Org. Lett. 2012, 14, 672.
(b) Miyake, Y.; Nakajima, K.; Nishibayashi, Y. J. Am. Chem. Soc. 2012, 134, 3338.
(c) Espelt, L. R.; McPherson, I. S.; Wiensch, E. M.; Yoon, T. P. J. Am. Chem. Soc. 2015, 137, 2452.
[9] Tan, Y. Q.; Yuan, W.; Gong, L.; Meggers, E. Angew. Chem., Int. Ed. 2015, 54, 13045.
[10] (a) Maity, S.; Zhu, M. Z.; Shinabery, R. S.; Zheng, N. Angew. Chem., Int. Ed. 2012, 51, 222.
(b) Nguyen, T. H.; Maity, S.; Zheng, N. Beilstein J. Org. Chem. 2014, 10, 975.
(c) Staveness, D.; Sodano, T. M.; Li, K. J.; Burnham, E. A.; Jackson, K. D.; Stephenson, C. R. J. Chem 2019, 5, 1.
[11] (a) Russell, G. A.; Weiner, S. A. J. Am. Chem. Soc. 1967, 89, 6623.
(b) Ma, J. J.; Rosales, A. R.; Huang, X. Q.; Harms, K.; Riedel R.; Wiest, O.; Meggers, E. J. Am. Chem. Soc. 2017, 139, 17245.
[12] Prier, C. K.; Rankic, D. A.; MacMillan, D. W. C. Chem. Rev. 2013, 113, 5322.
[13] Reger, D.; Haines P.; Heinemann, F. W.; Guldi, D. M.; Jux, N. Angew. Chem., Int. Ed. 2018, 57, 5938.
[14] Cui, W.; Loeppky, R. N. Tetrahedron 2001, 57, 2953.
/
| 〈 |
|
〉 |