

Chinese Journal of Organic Chemistry >
Recent Progress on Reactions of Arylmethyl Azides with Alkenes
Received date: 2019-03-01
Revised date: 2019-03-30
Online published: 2019-04-16
Supported by
Project supported by the National Natural Science Foundation of China (No. 21761033), the Natural Science Foundation of Guangxi (Nos. 2017GXNSFBA198211, 2018GXNSFAA294064), and the Yulin Normal University Research (Nos. 2018YJKY36, 201810606010).
Arylmethyl azides (ArCH2N3) as one of the significant nitrogen sources with stable properties, simple synthesis, have been widely used in a wide range of organic synthesis reactions. The recent progress (2014~2018) on reactions of arylmethyl azides with alkenes is summarized. In addition, the organic reactions of arylmethyl azides with types of alkenes are described respectively, with their scope of substrates and reaction mechanism. It is hoped that this review can be referred to the future application in organic synthesis of arylmethyl azides with alkenes.
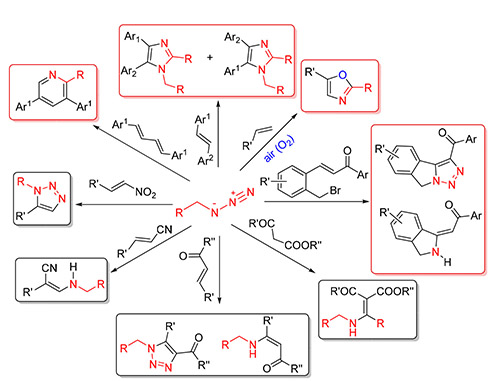
Key words: arylmethyl azides; alkenes; reaction mechanism
Li Xiuying , Li Yajun , Wei Xiansheng , Luo Jinrong , Huang Guobao , Tan Minxiong . Recent Progress on Reactions of Arylmethyl Azides with Alkenes[J]. Chinese Journal of Organic Chemistry, 2019 , 39(7) : 1831 -1836 . DOI: 10.6023/cjoc201903001
[1] Grecianl, S.; Aubé, J. Organic Azides:Syntheses and Applications, Vol. 7, Eds.:Bräse, S.; Banert, K., John Wiley & Sons, Ltd, Chichester, UK, 2010, pp. 191~310.
[2] (a) Bräse, S.; Gil, C.; Knepper, K.; Zimmermann, V. Angew. Chem., Int. Ed. 2005, 44, 5188.
(b) Lee, J. H.; Gupta, S.; Jeong, W.; Rhee, Y. H.; Park, J. Angew. Chem., Int. Ed. 2012, 51, 10851.
(c) Han, J.; Jeon, M.; Pak, H. K.; Rhee, Y. H.; Park, J. Adv. Synth. Catal. 2014, 356, 2769.
(d) Gupta, S.; Han, J.; Kim, Y.; Lee, S. W.; Rhee, Y. H.; Park. J. J. Org. Chem. 2014, 79, 9094.
(e) Chou, H.-H.; Raines, R. T. J. Am. Chem. Soc. 2013, 135, 14936.
(f) Zhang, X.-X.; Sun, X.-P.; Zhang, H.-F.; Cui, X.-L.; Ma, M.-T. Chin. J. Org. Chem. 2015, 35, 1469(in Chinese). (张小祥, 孙小萍, 张海飞, 崔杏丽, 马猛涛, 有机化学, 2015, 35, 1469.)
(g) Zhang, W.-S.; Xu, W.-J.; Kuang, C.-X. Chin. J. Org. Chem. 2015, 35, 2059(in Chinese). (张文生, 许文静, 匡春香, 有机化学, 2015, 35, 2059.)
[3] (a) Song, Z.-Q.; Zhao, Y.-M.; Zhai, H.-B. Org. Lett. 2011, 13, 6331.
(b) Lamani, M.; Devadig, P.; Prabhu, K. R. Org. Biomol. Chem. 2012, 10, 2753.
(c) Tummatorn, J.; Thongsornkleeb, C.; Ruchirawat, S.; Gettongsong, T. Org. Biomol. Chem. 2013, 11, 1463.
[4] Shin, K.; Kim, H.; Chang, S. Acc. Chem. Res. 2015, 48, 1040.
[5] Li, J.-L.; Wang, Y.-C.; Li, W.-Z.; Wang, H.-S.; Mo, D.-L.; Pan, Y.-M. Chem. Commun. 2015, 51, 17772.
[6] Tummatorn, J.; Poonsilp, P.; Nimnual, P.; Janprasit, J.; Thongsornkleeb, C.; Ruchirawat, S. J. Org. Chem. 2015, 80, 4516.
[7] Wang, Y.-C.; Li, J.-L.; He, Y.; Xie, Y.-Y.; Wang, H.-S.; Pan, Y.-M. Adv. Synth. Catal. 2015, 357, 3229.
[8] Wang, Y.-C.; Xie, Y.-Y.; Qu, H.-E.; Wang, H.-S.; Pan, Y.-M.; Huang, F. -P. J. Org. Chem. 2014, 79, 4463.
[9] Zefirov, N. S.; Chapovskaya, N. K.; Kolesnikov, V. V. Chem. Commun. 1971, 1001.
[10] Piet, J. C.; Le H. G.; Cailleux, P.; Benhaoua, H.; Carrie, R. Bull. Soc. Chim. Belg. 1996, 105, 33.
[11] Amantini, D.; Fringuelli, F.; Piermatti, O.; Pizzo, F.; Zunino, E.; Vaccaro, L. J. Org. Chem. 2005, 70, 6526.
[12] Wang, Y.-C.; Xie, Y.-Y.; Tan, X.-C.; Wang, H.-S.; Pan, Y.-M. Org. Biomol. Chem. 2015, 13, 513.
[13] Donald, A. S. R.; Marks, R. E. J. Chem. Soc. C 1967, 1188.
[14] Casey, M.; Donnelly, J. A.; Ryan, J. C.; Ushioda, S. ARKIVOC 2003, 7, 310.
[15] (a) Reddy, D. S.; Judd, W. R.; Aubé, J. Org. Lett. 2003, 5, 3899.
(b) Silvio, C.; Amenson, T. G. Tetrahedron Lett. 2012, 53, 6710.
[16] Mahoney, J. M.; Smith, C. R.; Johnston, J. N. J. Am. Chem. Soc. 2005, 127, 1354.
[17] Jumreang, T.; Charnsak, T.; Somsak, R.; Tanita, G. Org. Biomol. Chem. 2013, 11, 1463.
[18] Xie, Y.-Y.; Wang,Y.-C.; Qu, H.-E.; Tan, X.-C.; Wang, H.-S.; Pan, Y.-M. Adv. Synth. Catal. 2014, 356, 3347.
[19] Gangaprasad, D.; Paul Raj, J.; Kiranmye, T.; Sagubar Sadik, S.; Elangovan, J. RSC Adv. 2015, 5, 63473.
[20] Yang, W.-C.; Miao, T.; Li, P.-H.; Wang, L. RSC Adv. 2015, 5, 95833.
[21] Gangaprasad, D.; Paul Raj, J.; Kiranmye, T.; Karthikeyan, K.; Elangovan, J. Eur. J. Org. Chem. 2016, 5642.
[22] Xie, Y.-Y.; Wang, Y.-C.; He, Y.; Hu, D.-C.; Wang, H.-S.; Pan, Y.-M. Green Chem. 2017, 19, 656.
[23] Yan, Z.-M.; Wu, N.; Liang, D.; Wang, H.-S.; Pan, Y.-M. Org. Lett. 2014, 16, 4048.
/
| 〈 |
|
〉 |