

Chinese Journal of Organic Chemistry >
Synthesis and Antitumor Activity Evaluation of 2,4-Substituted Py-rimidine Derivatives Containing Trifluoromethyl
Received date: 2019-03-13
Revised date: 2019-04-16
Online published: 2019-04-26
Supported by
Project supported by the National Natural Science Foundation of China (No. 81430085) and the Natural Science Foundation of Henan Province (No. 182300410321).
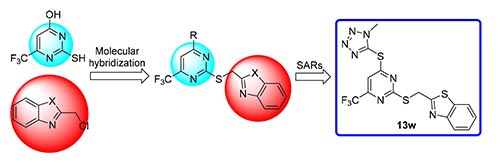
In order to find more effective antitumor drugs, a series of novel 2,4-substituted pyrimidine derivatives containing trifluoromethyl were designed, synthesized, and evaluated for antitumor activity aganist EC-109 (human esophageal cancer cell), MGC-803 (human gastric cancer cell), PC-3 (human prostate cancer cell) and HepG-2 (human liver cancer cell). The results showed that some compounds displayed moderate to potent antitumor activity against PC-3. Among them, 2-(((4-((1-methyl-1H-tetrazol-5-yl)thio)-6-(trifluoromethyl)pyrimidin-2-yl)thio)methyl)benzo[d]thiazole (13w) possesses strong antitu-mor activity against PC-3 with IC50 value of 1.76 μmol·L-1, and the antitumor activity is significantly better than the positive control drug of 5-fluorouracil.
Meng Yaqi , Li Erdong , Zhang Yang , Liu Shuan , Bao Chongnan , Yang Peng , Zhang Luye , Zhang Danqing , Wang Jikuan , Chen Yaxin , Li Na , Xin Jingchao , Zhao Peirong , Ke Yu , Zhang Qiurong , Liu Hongmin . Synthesis and Antitumor Activity Evaluation of 2,4-Substituted Py-rimidine Derivatives Containing Trifluoromethyl[J]. Chinese Journal of Organic Chemistry, 2019 , 39(9) : 2541 -2548 . DOI: 10.6023/cjoc201903022
[1] Addepalli, Y.; Yang, X.; Zhou, M.; Reddy, D. P.; Zhang, S. L.; Wang, Z.; He, Y. Eur. J. Med. Chem. 2018, 151, 214.
[2] Akhtar, J.; Khan, A. A.; Ali, Z.; Haider, R.; Shahar Yar, M. Eur. J. Med. Chem. 2017, 125, 143.
[3] Okesli, A.; Khosla, C.; Bassik, M. C. Curr. Opin. Biotechnol. 2017, 48, 127.
[4] Kahriman, N.; Serdaroglu, V.; Peker, K.; Aydin, A.; Usta, A.; Fandakli, S.; Yayli, N. Bioorg. Chem. 2019, 83, 580.
[5] Tageldin, G. N.; Fahmy, S. M.; Ashour, H. M.; Khalil, M. A.; Nassra, R. A.; Labouta, I. M. Bioorg. Chem. 2018, 78, 358.
[6] Li, Z. H.; Liu, X. Q.; Zhao, T. Q.; Geng, P. F.; Guo, W. G.; Yu, B.; Liu, H. M. Eur. J. Med. Chem. 2017, 139, 741.
[7] Abdellatif, K. R. A.; Bakr, R. B. Bioorg. Chem. 2018, 78, 341.
[8] Kuppast, B.; Fahmy, H. Eur. J. Med. Chem. 2016, 113, 198.
[9] Zhang, H.; Wang, J.; Shen, Y.; Wang, H. Y.; Duan, W. M.; Zhao, H. Y.; Hei, Y. Y.; Xin, M.; Cao, Y. X.; Zhang, S. Q. Eur. J. Med. Chem. 2018, 148, 221.
[10] Jabbour, E. Am. J. Hematol. 2016, 91, 59
[11] Usachev, B. I. J. Fluorine Chem. 2018, 210, 6.
[12] Kaur, K.; Kumar, V.; Gupta, G. K. J. Fluorine Chem. 2015, 178, 306.
[13] Xin, M.; Zhang, L.; Wen, J.; Shen, H.; Liu, Z.; Zhao, X.; Jin, Q.; Wang, M.; Cheng, L.; Huang, W.; Tang, F. Bioorg. Med. Chem. 2016, 24, 1079.
[14] Carmine, A. A.; Broden R. N.; Heel, R. C.; Speight, T. M.; Avery, G. S. Drugs 1982, 23, 329.
[15] Xie, X.; Yan, Y.; Zhu, N.; Liu, G. Eur. J. Med. Chem. 2014, 76, 67.
[16] Abdelgawad, M. A.; Bakr, R. B.; Omar, H. A. Bioorg. Chem. 2017, 74, 82.
[17] Akhtar, W.; Khan, M. F.; Verma, G.; Shaquiquzzaman, M.; Rizvi, M. A.; Mehdi, S. H.; Akhter, M.; Alam, M. M. Eur. J. Med. Chem. 2017, 126, 705.
[18] Li, N.; Xin, J. C.; Meng, Y, Q.; Li, E, D.; Ma, Q, S.; Bao, C, N.; Yang, P.; Song, P, P.; Cui, F.; Zhao, P, R.; Li, W.; Ke, Y.; Zhang, Q, R.; Liu, H, M. Chin. J. Org. Chem. 2018, 38, 2673.
[19] Nepali, K.; Sharma, S.; Sharma, M.; Bedi, P. M.; Dhar, K. L. Eur. J. Med. Chem. 2014, 77, 422.
[20] Kerru, N.; Singh, P.; Koorbanally, N.; Raj, R.; Kumar, V. Eur. J. Med. Chem. 2017, 142, 179.
[21] Lokwani, D.; Azad, R.; Sarkate, A.; Reddanma, P.; Shinde, D. Bioorg. Med. Chem. 2015, 23, 4533.
[22] Chen, P. J.; Yang, A.; Gu, Y. F.; Zhang, X. S.; Shao, K. P.; Xue, D. Q.; He, P.; Jiang, T. F.; Zhang, Q. R.; Liu, H. M. Bioorg. Med. Chem. Lett. 2014, 24, 2741.
[23] Hao, Y.; Chen, Y. Dyes Pigm. 2016, 129, 186.
[24] Gellis, A.; Boufatah, N.; Vanelle, P. Green Chem. 2006, 8, 483.
/
| 〈 |
|
〉 |