

Chinese Journal of Organic Chemistry >
Chiral Spiro Dienes Derived Boranes for Asymmetric Hydrosilylation of Ketones
Received date: 2019-03-30
Revised date: 2019-04-24
Online published: 2019-05-10
Supported by
Project supported by the National Natural Science Foundation of China (No. 21825108).
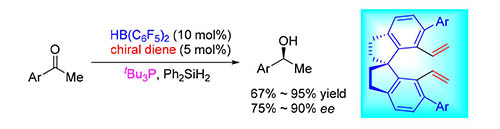
The chemistry of frustrated Lewis pairs (FLPs) is among the challenging frontiers of synthetic chemistry, which provides a powerful approach for metal-free catalytic hyrogenations and Piers-type hydrosilylations. In recent years, a significant progress has been made in this field. However, the deveopment of asymmetric reactions is still sluggish. The lacks of highly effective and enantioselective chiral FLP catalysts represent the key issue. C2-symmetric 1,1'-spirobiindane is one privileged framework in chiral ligands and catalysts. On the basis of chiral binaphthyl diene-derived frustrated Lewis pairs (FLPs) developed by our group, in this work, we designed and synthesized a novel class of chiral spiro dienes, which could further react with Piers' borane via the hydroboration reaction to generate chiral boranes in situ. With the combination of chiral borane and tri-tert-butylphosphine as an FLP catalyst, an asymmetric Piers-type hydrosilylation of simple ketones was successfully realized to give the desired secondary alcohols with up to 90% ee.
Wang Qiaotian , Han Caifang , Feng Xiangqing , Du Haifeng . Chiral Spiro Dienes Derived Boranes for Asymmetric Hydrosilylation of Ketones[J]. Chinese Journal of Organic Chemistry, 2019 , 39(8) : 2257 -2263 . DOI: 10.6023/cjoc201903076
[1] Welch, G. C.; San Juan, R. R.; Masuda, J. D.; Stephan, D. W. Science 2006, 314, 1124.
[2] (a) Stephan, D. W. Org. Biomol. Chem. 2008, 6, 1535.
(b) Kenward, A. L.; Piers, W. E. Angew. Chem., Int. Ed. 2008, 47, 38.
(c) Stephan, D. W.; Erker, G. Angew. Chem., Int. Ed. 2010, 49, 46.
(d) Soös, T. Pure Appl. Chem. 2011, 83, 667.
(e) Erker, G. Pure Appl. Chem. 2012, 84, 2203.
(f) Stephan, D. W. Org. Biomol. Chem. 2012, 10, 5740.
(g) Paradies, J. Angew. Chem., Int. Ed. 2014, 53, 3552.
(h) Stephan, D. W. Acc. Chem. Res. 2015, 48, 306.
(i) Stephan, D. W.; Erker, G. Angew. Chem., Int. Ed. 2015, 54, 6400.
(j) Oestreich, M.; Hermeke, J.; Mohr, J. Chem. Soc. Rev. 2015, 44, 2202.
(k) Stephan, D. W. Science 2016, 354, aaf7229.
[3] (a) Liu, Y.; Du, H. Acta Chim. Sinica 2014, 72, 771(in Chinese). (刘勇兵,杜海峰,化学学报, 2014, 72, 771.)
(b) Feng, X.; Du, H. Tetrahedron Lett. 2014, 55, 6959.
(c) Shi, L.; Zhou, Y.-G. ChemCatChem 2015, 7, 54.
(d) Meng, W.; Feng, X.; Du, H. Acc. Chem. Res. 2018, 51, 191.
[4] (a) Sumerin, V.; Chernichenko, K.; Nieger, M.; Leskelä, M.; Rieger, B.; Repo, T. Adv. Synth. Catal. 2011, 353, 2093.
(b) Mewald, M.; Fröhlich, R.; Oestreich, M. Chem.-Eur. J. 2011, 17, 9406.
(c) Mewald, M.; Oestreich, M. Chem.-Eur. J. 2012, 18, 14079.
(d) Lindqvist, M.; Borre, K.; Axenov, K.; Kótai, B.; Nieger, M.; Leskelä, M.; Pápai, I.; Repo, T. J. Am. Chem. Soc. 2015, 137, 4038.
(e) Süsse, L.; Hermeke, J.; Oestreich, M. J. Am. Chem. Soc. 2016, 138, 6940.
(f) Lam, J.; Günther, B. A. R.; Farrell, J. M.; Eisenberger, P.; Bestvater, B. P.; Newman, P. D.; Melen, R. L.; Crudden, C. M.; Stephan, D. W. Dalton Trans. 2016, 45, 15303.
[5] (a) Chen, D.; Wang, Y.; Klankermayer, J. Angew. Chem., Int. Ed. 2010, 49, 9475.
(b) Chen, D.; Leich, V.; Pan, F.; Klankermayer, J. Chem.-Eur. J. 2012, 18, 5184.
(c) Ghattas, G.; Chen, D.; Pan, F.; Klankermayer, J. Dalton Trans. 2012, 41, 9026.
(d) Ye, K.-Y.; Wang, X.; Daniliuc, C. G.; Kehr, G.; Erker, G. Eur. J. Inorg. Chem. 2017, 368.
(e) Chen, D.; Klankermayer, J. Chem. Commun. 2008, 2130.
[6] (a) Parks, D. J.; Spence, R. E. von H.; Piers, W. E. Angew. Chem., Int. Ed. 1995, 34, 809.
(b) Parks, D. J.; Piers, W. E.; Yap, G. P. A. Organometallics 1998, 17, 5492.
[7] (a) Liu, Y.; Du, H. J. Am. Chem. Soc. 2013, 135, 12968.
(b) Wei, S.; Du, H. J. Am. Chem. Soc. 2014, 136, 12261.
(c) Zhang, Z.; Du, H. Angew. Chem., Int. Ed. 2015, 54, 623.
(d) Ren, X.; Du, H. J. Am. Chem. Soc. 2016, 138, 810.
(e) Liu, X.; Wang, Q.; Han, C.; Feng, X.; Du, H. Chin. J. Chem. 2019, 37, 663.
[8] (a) Tu, X.-S.; Zeng, N.-N.; Li, R.-Y.; Zhao, Y.-Q.; Xie, D.-Z.; Peng Q.; Wang, X.-C. Angew. Chem., Int. Ed. 2018, 57, 15096.
(b) Li, X.; Tian, J.-J.; Liu, N.; Tu, X.-S.; Zeng, N.-N.; Wang, X.-C. Angew. Chem., Int. Ed. 2019, 58, 4664.
[9] Zhou, Q.-L., Privileged Chiral Ligands and Catalysts, Wiley-VCH, Weinheim, Germany, 2011.
[10] For selected reviews, see:(a) Xie, J.-H.; Zhou, Q.-L. Acc. Chem. Res. 2008, 41, 581.
(b) Ding, K.-L.; Han, Z.-B.; Wang, Z. Chem. Asian J. 2009, 4, 32.
(c) Xie, J.-H.; Zhou, Q.-L. Acta Chim. Sinica 2014, 72, 778(in Chinese). (谢建华,周其林,化学学报, 2014, 72, 778.)
(d) Xie, J.-H.; Bao, D.-H.; Zhou, Q.-L. Synthesis 2015, 47, 460.
(d) Zhu, S.-F.; Zhou, Q.-L. Acc. Chem. Res. 2012, 45, 1365.
[11] For a pioneering work, see:Parks, D. J.; Piers, W. E. J. Am. Chem. Soc. 1996, 118, 9440.
[12] Rendler, S.; Oestreich, M. Angew. Chem., Int. Ed. 2008, 47, 5997.
[13] Zhu, S.-F.; Yang, Y.; Wang, L.-X.; Liu, B.; Zhou, Q.-L. Org. Lett. 2005, 7, 2333.
[14] Zheng, J.; Cui, W.-J.; Zheng, C.; You, S.-L. J. Am. Chem. Soc. 2016, 138, 5242.
[15] For details, see the Supporting Information. CCDC 1905630 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
[16] (a) Sokeirik, Y. S.; Mori, H.; Omote, M; Sato, K.; Tarui, A.; Kumadaki, I.; Ando, A. Org. Lett. 2007, 9, 1927.
(b) Wu, W.; Liu, S.; Duan, M.; Tan, X.; Chen, C.; Xie, Y.; Lan, Y.; Dong, X.; Zhang, X. Org. Lett. 2016, 18, 2938.
(c) Ren, X.; Li, G.; Wei, S.; Du, H. Org. Lett. 2015, 17, 990.
(d) Zhang, Z.; Jain, P.; Antilla, J. C. Angew. Chem., Int. Ed. 2011, 50, 10961.
(e) Süsse, L.; Hermeke, J.; Oestreich, M. J. Am. Chem. Soc. 2016, 138, 6940.
(f) Chen, X.; Lu, Z. Org. Lett. 2016, 18, 4658.
/
| 〈 |
|
〉 |