

Chinese Journal of Organic Chemistry >
Asymmetric 1,3-Dipolar Cycloaddition Reaction of C,N-Diarylnitrone with N-α,β-Unsaturated Acyl Compounds Catalyzed by Chiral Bisoxazoline Metal Complex
Received date: 2019-01-04
Revised date: 2019-03-02
Online published: 2019-05-15
Supported by
Project supported by the Natural Science Foundation of Heilongjiang Province (No. LH2019B010), and the National Natural Science Foundation of China (No. 21506043).
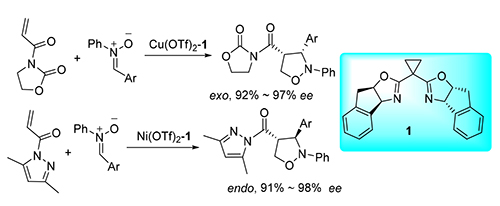
Asymmetric cycloaddition reactions catalyzed by Inda-BOX 1 metal complex between two kinds of electron-withdrawing alkenes and C,N-diarylnitrone have been studied respectively. Results showed that the 4-substituted products were mainly obtained in both reactions under the best conditions. When N-acryloyl oxazolidinone was used as dipolarophile, the exo/endo selectivity of the reaction was 100/0, and the ee of the exo product was as high as 97%. When N-acryloyl-3,5-dimethyl pyrazole was used as dipolarophile, the selectivity of exo/endo was 0/100, and the ee of endo product was up to 98%. The relationship of the dipolarophile, the structure of nitrone and the selectivity of the reaction was discussed.
Li Minglong , Cao Xixian , You Jun , Yu Yanchao , Wu Wenju , Liu Bo . Asymmetric 1,3-Dipolar Cycloaddition Reaction of C,N-Diarylnitrone with N-α,β-Unsaturated Acyl Compounds Catalyzed by Chiral Bisoxazoline Metal Complex[J]. Chinese Journal of Organic Chemistry, 2019 , 39(6) : 1642 -1649 . DOI: 10.6023/cjoc201901004
[1] Gothelf, K.-V.; Jørgensen, K.-A.; Benito, A. Chem. Rev. 1998, 98, 863.
[2] Chiacchio, U.; Corsaro, A.; Iannazzo, D.; Piperno, A.; Pistarà, V.; Rescifina, A.; Romeo, R.; Valveri, V.; Mastino, A.; Romeo, G. J. Med. Chem. 2003, 46, 3696.
[3] Karanjule, N.-S.; Markad, S.-D.; Sharma, T; Sabharwal, S.-G.; Puranik, V.-G.; Dhavale, D.-D. J. Org. Chem. 2005, 70, 1356.
[4] Stanley, L.-M.; Sibi, M.-P. Chem. Rev. 2008, 108, 2887.
[5] Wang, D.-H.; Wang, F.; Chen, P.-H.; Lin, Z.-Y.; Liu, G.-S. Angew. Chem., Int. Edit. 2017, 56, 2054.
[6] Adachi, S.; Takeda, N.; Sibi, M.-P. Org. Lett. 2014, 16, 6440.
[7] Sibi, M.-P.; Yang, Y.-H.; Lee, S. Org. Lett. 2008, 10, 5349.
[8] Shibata, S.; Ikeda, M.; Motoyama, K.; Miyake, Y.; Nishibayashi, Y. Chem. Commun. 2012, 48, 9528.
[9] Hofstra, J.-L.; Cherney, A.-H.; Ordner, C.-M.; Reisman, S.-E. J. Am. Chem. Soc. 2018, 140, 139.
[10] Suzuki, N.; Hofstra, J.-L.; Poremba, K.-E.; Reisman, S.-E. Org. Lett. 2017, 19, 2150.
[11] Sibi, M.-P.; Ma, Z.-H.; Itoh, K.; Prabagaran, N.; Jasperse, C.-P. Org. Lett. 2005, 7, 2349.
[12] Sibi, M.-P.; Stanley, L.-M.; Jasperse, C.-P. J. Am. Chem. Soc. 2005, 127, 8276.
[13] Sibi, M.-P.; Rane, D.; Stanley, L.-M.; Soeta, T. Org. Lett. 2008, 10, 2971.
[14] Ishihara, K.; Fushimi, M. Org. Lett. 2006, 8, 1921.
[15] Minakata, S.; Murakami, Y.; Tsuruoka, R.; Kitanaka, S.; Komatsu, M. Chem. Commun. 2008, 6363.
[16] Ono, F.; Ohta, Y.; Hasegawa, M.; Kanemasa, S. Tetrahedron Lett. 2009, 50, 2111.
[17] Sakakura, A.; Hori, M.; Fushimi, M.; Ishihara, K. J. Am. Chem. Soc. 2010, 132, 15550.
[18] Narasaka, K.; Kusama, K.; Hayashi, Y. Bull. Chem. Soc. Jpn. 1991, 64, 1471.
[19] Takenaka, N.; Huang, Y.; Rawal, V.-H. Tetrahedron. 2002, 58, 8299.
[20] Sibi, M.-P.; Zhang, R.-H.; Manyem, S. J. Am. Chem. Soc. 2003, 125, 9306.
[21] Wang, W.-T.; Rein, K.-S. Tetrahedron Lett. 2013, 54, 1866.
[22] Jensen, K.-B.; Gothelf, K.-V.; Jorgensen, K.-A. Helv. Chim. Acta 1997, 80, 2039.
[23] Liu, X.-S.; Li, M.-M.; You, J.; Liu, B. Chin. J. Org. Chem. 2017, 37, 86(in Chinese).
[24] Saito, T.; Yamada, T.; Miyazaki, S.; Otani, T. Tetrahedron Lett. 2004, 45, 9585.
[25] Iwasa, S.; Tsushima, S.; Shimada, T.; Nishiyama, H. Tetrahedron Lett. 2001, 42, 6715.
[26] Desimoni, G.; Faita, G.; Toscanini, M.; Boiocchi, M. Chem.-Eur. J. 2009, 15, 9674.
[27] Gerten, A.-L.; Slade, M.-C.; Pugh, K.-M.; Stanley, L.-M. Org. Biomol. Chem. 2013, 11, 7834.
[28] Sibi, M.-P.; Itoh, K.; Jasperse, C.-P. J. Am. Chem. Soc. 2004, 126, 5366.
[29] Hu, H.-X.; Wang, Y.-D.; Qian, D.-Y.; Zhang, Z.-M.; Liu, L.; Zhang, J.-L. Org. Chem. Front. 2016, 3, 759.
[30] Zhu, Y.-K.; Liu, B.; You, J.; Wang Y.-X. J. Chem. Res. 2007, 11, 662.
[31] Kashima, C.; Takahashi, K.; Fukuchi, I. Heterocycles 1997, 1, 289.
/
| 〈 |
|
〉 |