

Chinese Journal of Organic Chemistry >
Diversified Synthesis of Imines via Aerobic Oxidation Catalyzed by Dinuclear Butterfly-Like Cu(I) Complex
Received date: 2019-03-15
Revised date: 2019-04-03
Online published: 2019-05-15
Supported by
Project supported by the National Natural Science Foundation of China (No. 21402112), and the 1331 Project of Shanxi Province.
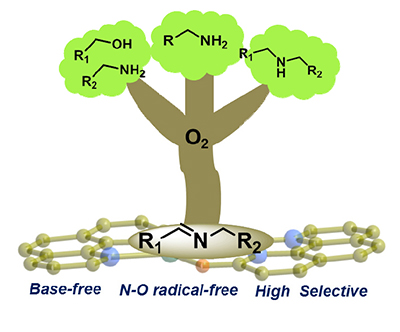
The selective catalytic oxidation of amines for the synthesis of imines is important both in laboratory and industrial production. From atom-efficient, economic and environmental view of points, dioxygen selective oxidation of amines was achieved by using wings-opened butterfly-like complex Cu2(ophen)2 as catalyst. It was worth noting that the catalytic system was efficient to the cross-coupling of alcohols with amines, homocoupling of primary amines and oxidative dehydrogenation of secondary amines. The yield is up to 93% and the selectivity of imines is as high as 99%. Avoiding the use of expensive nitroxyl derivatives and base was suitable for practical application.
Zhang Lingjuan , Dang Yujiao , Zhang Xianming . Diversified Synthesis of Imines via Aerobic Oxidation Catalyzed by Dinuclear Butterfly-Like Cu(I) Complex[J]. Chinese Journal of Organic Chemistry, 2019 , 39(6) : 1650 -1654 . DOI: 10.6023/cjoc201903027
[1] (a) Largeron, M. Eur. J. Org. Chem. 2013, 2013, 5225.
(b) Kobayashi, S.; Mori, Y.; Fossey, J. S.; Salter, M. M. Chem. Rev. 2011, 111 (4), 2626.
(c) Chen, B.; Wang, L.; Gao, S. ACS Catal. 2015, 5851.
(d) Zhang, Z. G.; Li, J. L.; Huang, G. Q.; Sun, K. Zhang, G. S.; Ma, N. N.; Liu, Q. F.; Liu, T. X. Chin. J. Chem. 2016, 34, 1309.
(e) Patil, R. D.; Adimurthy, S. Asian J. Org. Chem. 2013, 2, 726.
[2] (a) Mukherjee, A.; Nerush, A.; Leitus, G.; Shimon, L. J. W.; Ben David, Y.; Espinosa Jalapa, N. A.; Milstein, D. J. Am. Chem. Soc. 2016, 138, 4298.
(b) Chen, B.; Li, J.; Dai, W.; Wang, L.; Gao, S. Green Chem. 2014, 16, 332.
(c) Zhang, G.; Hanson, S. K. Org. Lett. 2013, 15, 650.
(d) Zhang, E.; Tian, H.; Xu, S.; Yu, X.; Xu, Q. Org. Lett. 2013, 15 (11), 2704.
(e) Montag, M.; Zhang, J.; Milstein, D. J. Am. Chem. Soc. 2012, 134 (25), 10325.
(f) Maggi, A.; Madsen, R. Organometallics 2012, 31, 451.
(g) He, W.; Wang, L.; Sun, C.; Wu, K.; He, S.; Chen, J.; Wu, P.; Yu , Z. Chem.-Eur. J. 2011, 17, 13308.
(h) Kegnaes, S.; Mielby, J.; Mentzel, U. V.; Christensen, C. H.; Riisager, A. Green Chem. 2010, 12, 1437.
(i) Jaiswal, G.; Landge, V. G.; Jagadeesan, D.; Balaraman, E. Green Chem. 2016, 18, 3232.
(j) Tamura, M.; Tomishige, K. Angew. Chem., Int. Ed. 2015, 54, 864.
(k) Saranya, S.; Ramesh, R.; Grzegorz Malecki, J. Eur. J. Org. Chem. 2017, 2017, 6726.
(l) Chandra, M. D.; Sadhukha, A.; Maayan, G. J. Catal. 2017, 355, 139.
(m) Han, L.; Xing, P.; Jiang, B. Org. Lett. 2014, 16, 3428.
[3] (a) Wang, Z.; Lang, X. Appl. Catal. B-Environ. 2018, 224, 404.
(b) Rodríguez-Lugo, R. E.; Chacón-Terán, M. A.; De León, S.; Vogt, M.; Rosenthal, A. J.; Landaeta, V. R. Dalton Trans. 2018, 47, 2061.
(c) Jin, J.; Yang, C.; Zhang, B.; Deng, K. J. Catal. 2018, 361, 33.
(d) Xu, B.; Hartigan, E. M.; Feula, G.; Huang, Z.; Lumb, J.-P.; Arndtsen, B. A. Angew. Chem., Int. Ed. 2016, 55, 15802.
(e) Su, C.; Tandiana, R.; Tian, B.; Sengupta, A.; Tang, W.; Su, J.; Loh, K. P. ACS Catal. 2016, 3594.
(f) Raza, F.; Park, J. H.; Lee, H.-R.; Kim, H.-I.; Jeon, S.-J.; Kim, J.-H. ACS Catal. 2016, 2754.
(g) Sun, D.; Ye, L.; Li, Z. Appl. Catal. B-Environ. 2015, 164, 428.
(h) Johnson, J. A.; Luo, J.; Zhang, X.; Chen, Y.-S.; Morton, M. D.; Echeverría, E.; Torres, F. E.; Zhang, J. ACS Catal. 2015, 5, 5283.
(i) Wu, X.-F.; Petrosyan, A.; Ghochikyan, T. V.; Saghyan, A. S.; Langer, P. Tetrahedron Lett. 2013, 54, 3158.
(j) Furukawa, S.; Ohno, Y.; Shishido, T.; Teramura, K.; Tanaka, T. ACS Catal. 2011, 1, 1150.
(k) Varyani, M.; Khatri, P. K.; Jain, S. L. Tetrahedron Lett. 2016, 57, 723.
[4] Nikbakht, F.; Heydari, A. CR Chim. 2015, 18, 132.
[5] (a) Liu, Y.; Chen, Y.; Zhou, W.; Jiang, B.; Zhang, X.; Tian, G. Catal. Sci. Technol. 2018, 8, 5535.
(b) Dutta, I.; De, S.; Yadav, S.; Mondol, R.; Bera, J. K. J. Organomet. Chem. 2017, 849-850, 117.
(c) Bai, L.; Dang, Z. RSC Adv. 2015, 5, 10341.
(d) Wang, J.; Lu, S.; Cao, X.; Gu, H. Chem. Comm. 2014, 50, 5637.
(e) Ryland, B. L.; Stahl, S. S. Angew. Chem., Int. Ed. 2014, 53, 8824.
(f) Tian, H.; Yu, X.; Li, Q.; Wang, J.; Xu, Q. Adv. Synth. Catal. 2012, 354, 2671.
(g) Kang, Q.; Zhang, Y. Green Chem. 2012, 14, 1016.
[6] (a) Lan, Y.-S.; Liao, B.-S.; Liu, Y.-H.; Peng, S.-M.; Liu, S.-T. Eur. J.Org. Chem. 2013, 2013, 5160.
(b) Pérez, J. M.; Cano, R.; Yus, M.; Ramón, D. J. Eur. J. Org. Chem. 2012, 2012, 4548.
[7] (a) Huang, B.; Tian, H.; Lin, S.; Xie, M.; Yu, X.; Xu, Q. Tetrahedron Lett. 2013, 54, 2861.
(b) Sonobe, T.; Oisaki, K.; Kanai, M. Chem. Sci. 2012, 3, 3249.
[8] Zhang, L. J.; Liu, J.; Zhang, F. Q.; Zhang, X.-M. J. Catal. 2017, 354, 78.
[9] (a) Shen, Y.; Zhou, Y.; Jiang, L.; Ding, G.; Luo, L.; Zhang, Z.; Xie, X. Tetrahedron 2018, 74, 4266.
(b) Tao, C.; Wang, B.; Sun, L.; Liu, Z.; Zhai, Y.; Zhang, X.; Wang, J. OrgBioChem 2017, 15, 328.
(c) Ray, R.; Chandra, S.; Yadav, V.; Mondal, P.; Maiti, D.; Lahiri, G. K. Chem. Commun. 2017, 53, 4006.
(d) Liu, G.; Sun, L.; Liu, J.; Wang, F.; Guild, C. J. Mol. Catal. 2017, 440, 148.
(e) Pavel, O. D.; Goodrich, P.; Cristian, L.; Coman, S. M.; Pârvulescu, V. I.; Hardacre, C. Catal. Sci. Technol. 2015, 5, 2696.
[10] Patil, R. D.; Adimurthy, S. RSC Adv. 2012, 2, 5119.
[11] Zhang, X.-M.; Tong, M.-L.; Gong, M. L.; Lee, H.-K.; Luo, L.; Li, K. F.; Tong, Y.-X.; Chen, X.-M. Chem.-Eur. J. 2002, 8, 3187.
[12] Zhang, G. Q.; Hanson, S. K. Org. Lett. 2013, 15, 3650.
[13] Zhang, Y.; Lu, F.; Huang, R.; Zhang, H.; Zhao, J. Catal. Commun. 2016, 81, 10.
/
| 〈 |
|
〉 |