

Chinese Journal of Organic Chemistry >
Regioselective Synthesis of Substituted Pyrazoles via[3+2]/[2+3] Cyclization of Saturated Ketone with Hydrazine/Aldehyde Hydrazone
Received date: 2019-03-23
Revised date: 2019-05-14
Online published: 2019-05-21
Supported by
Project supported by the National Natural Science Foundation of China (No. 21572047) and the Plan for Scientific Innovation Talents of Henan Province (No. 184200510012).
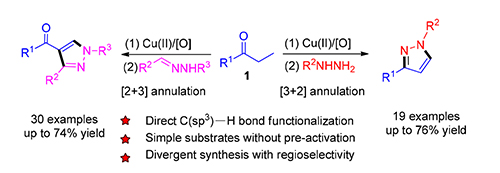
A highly convenient and regioselective synthesis of 1,3-disubstituted pyrazoles or 1,3,4-trisubstituted pyrazoles from Cu(Ⅱ)-catalyzed cascade reactions of saturated ketones with hydrazines or aldehyde hydrazones is presented. Mechanistically, the formation of 1,3-disubstituted pyrazoles involves the in situ generation of an enone intermediate followed by its[3+2] annulations with hydrazine. On the other hand, the formation of 1,3,4-trisubstituted pyrazoles is believed to go through a cascade process including enone formation and its subsequent[2+3] annulation with aldehyde hydrazone. Compared with literature methods, the notable features of the protocol include simple starting materials, general and broad substrate scope, high regioselectivity, good efficiency and excellent atom-economy.
Shi Xiaonan , Tian Miaomiao , Wang Muhua , Zhang Xinying , Fan Xuesen . Regioselective Synthesis of Substituted Pyrazoles via[3+2]/[2+3] Cyclization of Saturated Ketone with Hydrazine/Aldehyde Hydrazone[J]. Chinese Journal of Organic Chemistry, 2019 , 39(6) : 1630 -1641 . DOI: 10.6023/cjoc201903046
[1] (a) Fustero, S.; Sánchez-Roselló, M.; Barrio, P.; Simón-Fuentes, A. Chem. Rev. 2011, 111, 6984.
(b) Dadiboyena, S.; Nefzi, A. Eur. J. Med. Chem. 2011, 46, 5258.
(c) Wen, J.-J.; Zhu Y.; Zhan, Z.-P. Asian J. Org. Chem. 2012, 1, 108.
(d) Li, M.; Zhao, B.-X. Eur. J. Med. Chem. 2014, 85, 311.
(e) Küçükgüzel, S. G.; Senkardes, S. Eur. J. Med. Chem. 2015, 97, 786.
(f) Hughes, D. L. Org. Process Res. Dev. 2017, 21, 430.
(g) Faria, J. V.; Vegi, P. F.; Miguita, A. G. C.; dos Santos, M. S.; Boechat, N.; Bernardino, A. M. R. Bioorg. Med. Chem. 2017, 25, 5891.
[2] (a) Schmidt, A.; Dreger, A. Curr. Org. Chem. 2011, 15, 2897.
(b) Haydl, A. M.; Xu, K.; Breit, B. Angew. Chem., Int. Ed. 2015, 54, 7149.
(c) Fang, Z.; Liu, J.; Qiao, Y. Chin. J. Org. Chem. 2018, 38, 1985 (in Chinese).(房智兴, 刘巨艳, 乔艳红, 有机化学, 2018, 38, 1985.)
(d) Li, Y.; Dong, C.-E. Chin. Chem. Lett. 2015, 26, 623.
(e) Yu, X.; Zhang, J. Chem.-Eur. J. 2012, 18, 12945.
[3] (a) Zhang, X.; Kang, J.; Niu, P.; Wu, J.; Yu, W.; Chang, J. J. Org. Chem. 2014, 79, 10170.
(b) Sar, D.; Bag, R.; Yashmeen, A.; Bag, S. S.; Punniyamurthy, T. Org. Lett. 2015, 17, 5308.
(c) Sun, J.; Qiu, J.-K.; Zhu, Y.-L.; Guo, C.; Hao, W.-J.; Jiang, B.; Tu, S.-J. J. Org. Chem. 2015, 80, 8217.
(d) Ding, Y.; Zhang, T.; Chen, Q.-Y.; Zhu, C. Org. Lett. 2016, 18, 4206 and references cited therein.
(e) Muzalevskiy, V. M.; Rulev, A. Y.; Romanov, A. R.; Kondrashov, E. V.; Ushakov, I. A.; Chertkov, V. A.; Nenajdenko, V. G. J. Org. Chem. 2017, 82, 7200.
(f) Cernuchová, P.; Vo-Thanh, G.; Milata, V.; Loupy, A.; Jantová, S.; Theiszová, M. Tetrahedron 2005, 61, 5379.
[4] Chen, Z.; Zheng, Y.; Ma, J.-A. Angew. Chem., Int. Ed. 2017, 56, 4569.
[5] (a) Zhang, G.; Ni, H.; Chen, W.; Shao, J.; Liu, H.; Chen, B.; Yu, Y. Org. Lett. 2013, 15, 5967.
(b) Shu, W.-M.; Zheng, K.-L.; Ma, J.-R.; Sun, H.-Y.; Wang, M.; Wu, A.-X. Org. Lett. 2015, 17, 1914.
(c) Tu, Y.; Zhang, Z.; Wang, T.; Ke, J.; Zhao, J. Org. Lett. 2017, 19, 3466.
(d) Harigae, R.; Moriyama, K.; Togo, H. J. Org. Chem. 2014, 79, 2049.
[6] (a) Li, X.; He, L.; Chen, H.; Wu, W.; Jiang, H. J. Org. Chem. 2013, 78, 3636.
(b) Fan, X.-W.; Lei, T.; Zhou, C.; Meng, Q.-Y.; Chen, B.; Tung, C.-H.; Wu, L.-Z. J. Org. Chem. 2016, 81, 7127.
(c) Pünner, F.; Sohtome, Y.; Sodeoka, M. Chem. Commun. 2016, 52, 14093.
(d) Yang, Y.; Hu, Z.-L.; Li, R.-H.; Chen, Y.-H.; Zhan, Z.-P. Org. Biomol. Chem. 2018, 16, 197.
[7] Li, F.; Wang, J.; Pei, W.; Li, H.; Zhang, H.; Song, M.; Guo, L.; Zhang, A.; Liu, L. Tetrahedron Lett. 2017, 58, 4344.
[8] (a) Diao, T.; Pun, D.; Stahl, S. S. J. Am. Chem. Soc. 2013, 135, 8205.
(b) Pun, D.; Diao, T.; Stahl, S. S. J. Am. Chem. Soc. 2013, 135, 8213.
[9] (a) Shang, Y.; Jie, X.; Zhou, H.; Hu, P.; Huang, S.; Su, W. Angew. Chem., Int. Ed. 2013, 52, 1299.
(b) Gandeepan, P.; Rajamalli, P.; Cheng, C.-H. ACS Catal. 2014, 4, 4485.
(c) Jie, X.; Shang, Y.; Zhang, X.; Su, W. J. Am. Chem. Soc. 2016, 138, 5623.
(d) Zhan, J.-L.; Wu, M.-W.; Chen, F.; Han, B. J. Org. Chem. 2016, 81, 11994.
(e) Guo, W.; Liu, D.; Liao, J.; Ji, F.; Wu, W.; Jiang, H. Org. Chem. Front. 2017, 4, 1107.
[10] (a) Wang, Z.; Chen, G.; Zhang, X.; Fan, X. Org. Chem. Front. 2017, 4, 612.
(b) Tian, M.; Shi, X.; Zhang, X.; Fan, X. J. Org. Chem. 2017, 82, 7363.
(c) Chen, G.; Wang, Z.; Zhang, X.; Fan, X. J. Org. Chem. 2017, 82, 11230.
[11] CCDC 1857401 (3f) contains the crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
[12] (a) Li, H.; Liu, C.; Zhang, Y.; Sun, Y.; Wang, B.; Liu, W. Org. Lett. 2015, 17, 932.
(b) Neumann, J. J.; Suri, M.; Glorius, F. Angew. Chem., Int. Ed. 2010, 49, 7790.
(c) Sun, Z.; Khan, J.; Makowska-Grzyska, M.; Zhang, M.; Cho, J. H.; Suebsuwong, C.; Vo, P.; Gollapalli, D. R.; Kim, Y.; Joachimiak, A.; Hedstrom, L.; Cuny, G. D. J. Med. Chem. 2014, 57, 10544.
[13] Voronin, V. V.; Ledovskaya, M. S.; Gordeev, E. G.; Rodygin, K. S.; Ananikov, V. P. J. Org. Chem. 2018, 83, 3819.
[14] Tang, X.; Huang, L.; Yang, J.; Xu, Y.; Wu, W.; Jiang, H. Chem. Commun. 2014, 50, 14793.
[15] Shan, G.; Liu, P.; Rao, Y. Org. Lett. 2011, 13, 1746.
[16] Zhang G.; Zhao, Y.; Ge, H. Angew. Chem., Int. Ed. 2013, 52, 2559.
[17] Comas-Barcel, J.; Foster, R. S.; Fiser, B.; Gomez-Bengoa, E.; Harrity, J. P. A. Chem.-Eur. J. 2015, 21, 3257.
/
| 〈 |
|
〉 |