

Chinese Journal of Organic Chemistry >
Design, Synthesis and Antitumor Activity Evaluation of 4-Aminoquinazoline Derivatives Containing Urea Moiety
Received date: 2019-03-27
Revised date: 2019-05-20
Online published: 2019-06-03
Supported by
Project supported by the National Natural Science Foundation of China(81430085);the Natural Science Foundation of Henan Province(182300410321);the Technology Department Project of Henan Province(No. 182102310249)
In order to find new anti-tumor drugs with targeted therapeutic effect, a series of novel 4-aminoquinazoline derivatives bearing urea moiety were designed, synthesized and evaluated for antitumor activity against four human cancer cell lines of MCF-7, MGC-803, SW620 and A549 using methyl thiazolyl tetrazolium (MTT) assay. Most of the target compounds exhibited excellent anti-tumor activity against the four human tumor cell lines. Among them, 2-((4-((3,4,5-trimethoxyphenyl)- amino)quinazolin-2-yl)-thio)-N-((3,4,5-trimethoxyphenyl)carbamoyl)acetamide (10p) showed the best antitumor activity against MGC-803, SW620 and A549 cancer cell lines with IC50 values of (7.02±0.46), (6.00±0.78) and (7.04±1.11) μmol? L –1, respectively. Its activity was comparable to the positive control of gefitinib. Molecular docking showed that compound 10p could bind well with EGFR, suggesting that it could be a potential antitumor agent.
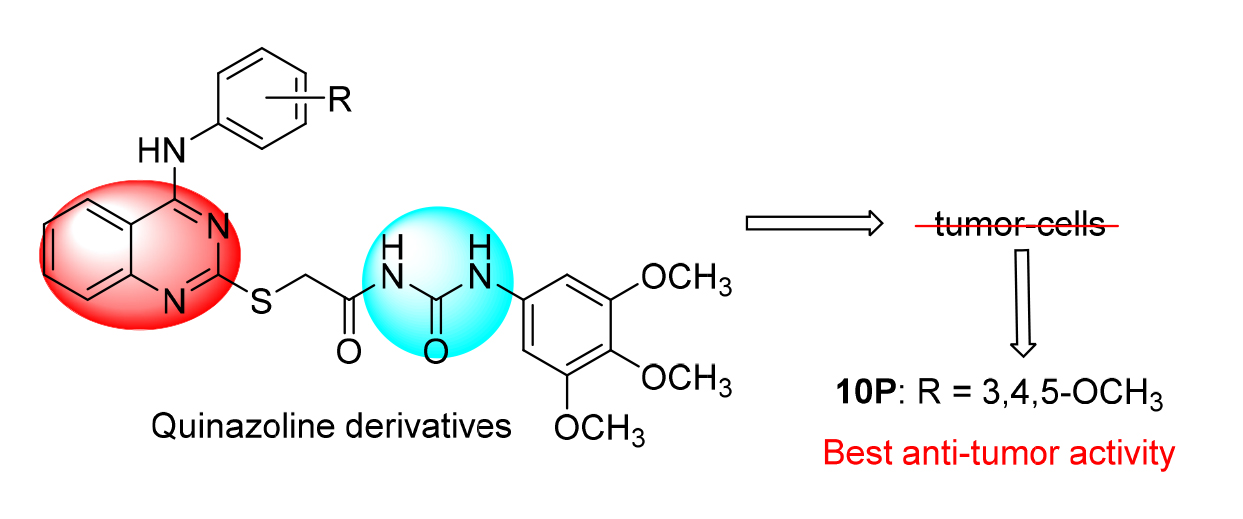
Key words: urea moiety; 4-aminoquinazoline; synthesis; antitumor activity
Erdong Li, , Yaqi Meng, , Luye Zhang, , Yang Zhang, , Jikuan Wang, , Danqing Zhang, , Panpan Song, , Jingchao Xin, , Na Li, , Jiaxin Zheng, , Yu Ke, , Hongmin Liu, , Qiurong Zhang, . Design, Synthesis and Antitumor Activity Evaluation of 4-Aminoquinazoline Derivatives Containing Urea Moiety[J]. Chinese Journal of Organic Chemistry, 2019 , 39(10) : 2875 -2881 . DOI: 10.6023/cjoc201903062
| [1] | Vainshelboim, B.; Müller, J.; Lima, R. M.; Chester, C.; Chan, K.; Myers, J . Preventive Med. 2017, 100, 89. |
| [2] | Lin, S. W.; Li, Y. B.; Zheng, Y. Y.; Li, L. C.; Sun, Q.; Ge, Z. M.; Cheng, T. M.; Li, R. T. Eur. J. Med. Chem. 2017, 127, 422. |
| [3] | Patel, T. S.; Vanparia, S. F.; Gandhi, S. A.; Patel, U. H.; Dixit, R. B.; Chudasama, C. J.; Dixit, B. C. New J. Chem. 2015, 39, 8638. |
| [4] | Alafeefy, A. M.; Kadi, A. A.; Al-Deeb, O. A.; El-Tahir, K. E. H.; Al-Jaber, N. A. Eur. J. Med. Chem. 2010, 45, 4947. |
| [5] | Cesar, M. M.; Jose C., B.; Rocío, N. M.; Adrian, M. N.; Rocío, A. S.; Miriam, M. C.; Benjamín, N. T.; Erick, S. C.; Alejandro, R. L.; Francisco, H. L. Eur. J. Med. Chem. 2015, 96, 296. |
| [6] | Mathew, M. P.; Tan, E.; Saeui, C. T.; Bovonratwet, P.; Liu, L.; Bhattacharya, R.; Yarema, K. J. Bioorg. Med. Chem. Lett. 2015, 25, 1223. |
| [7] | Zhang, Y.; Chen, L.; Xu, H.; Li, X.; Zhao, L.; Wang, W.; Zhang, X. Eur. J. Med. Chem. 2018, 147, 77. |
| [8] | Zhang, H. Q.; Gong, F. H.; Ye, J. Q.; Zhang, C.; Yue, X. H.; Li, C. G.; Sun, L. P. Eur. J. Med. Chem. 2017, 125, 245. |
| [9] | Qin, M.; Yan, S.; Wang, L.; Zhang, H.; Zhao, Y.; Wu, S.; Gong, P. Eur. J. Med. Chem. 2016, 115, 1. |
| [10] | Rekunge, D. S., Khatri, C. K., Chaturbhuj, G. U. Tetrahedron Lett. 2017, 58, 4304. |
| [11] | Borovlev, I. V.; Demidov, O. P.; Amangasieva, G. A.; Avakyan, E. K. Tetrahedron Lett. 2016, 57, 3608. |
| [12] | Tokala, R.; Bale, S.; Janrao, I. P.; Vennela, A.; Kumar, N. P.; Senwar, K. R.; Shankaraiah, N . Bioorg. Med. Chem. Lett. 2018, 28, 1919. |
| [13] | Keating, G. M . Targeted Oncol. 2017, 12, 243. |
| [14] | Kurt, B. Z.; Kandas, N. O.; Dag, A.; Sonmez, F.; Kucukislamoglu, M . Arabian J. Chem. 2017,10. |
| [15] | Li, N.; Xin, J. C.; Ma, Q. S.; Li, E. D.; Meng, Y. Q.; Bao, C. N.; Yang, P.; Song, P. P.; Cui, F.; Cheng, P. J.; Gu, Y. F.; Zhao, P. R.; Ke, Y.; Liu, H. M.; Zhang, Q. R. Chin. J. Org. Chem. 2018, 38, 665 (in Chinese). |
| [15] | ( 栗娜, 辛景超, 马启胜, 李二冬, 孟娅琪, 包崇男, 杨鹏, 宋攀攀, 崔飞, 陈鹏举, 顾一飞, 赵培荣, 可钰, 刘宏民, 张秋荣 , 有机化学, 2018, 38, 665.) |
| [16] | Song, P. P.; Cui, F.; Li, N.; Xin, J. C.; Ma, Q. S.; Meng, X. C.; Wang, C. J.; Cao, Q. P.; Gu, Y. F.; Ke, Y.; Zhang, Q. R.; Liu, H. M. Chin. J. Org Chem. 2017, 35, 1633 (in Chinese). |
| [16] | ( 宋攀攀, 崔飞, 栗娜, 辛景超, 马启胜, 孟祥川, 王超杰, 曹钦坡, 顾一飞, 可钰, 刘宏民, 张秋荣 , 有机化学, 2017, 35, 1633.) |
| [17] | Meng, X. C.; Li, N.; Li, E. D.; Chang, T. H.; Li, M.; Li, Q. Y.; Yuan, W. J.; Zhang, Q. Q.; Zhang, Y.; Zhou, Z. Y.; Song, P. P.; Liu, H. M.; Zhang, Q. R. Chin. J. Org. Chem. 2018, 38, 3063 (in Chinese). |
| [17] | ( 孟祥川, 栗娜, 李二冬, 常通航, 刘梦, 柳晴怡, 袁文娟, 张晴晴, 张钰, 周智玉, 宋攀攀, 刘宏民, 张秋荣, 有机化学, 2018, 38, 3063.) |
| [18] | Raboisson, P.; Marugán, J. J.; Schubert, C.; Koblish, H. K.; Lu, T.; Zhao, S.; Lattanze, J. Bioorg. Med. Chem. Lett. 2005, 15, 1857. |
| [19] | Renata, M.; Tomasz, M.; Jakub, R. Cell. Mol. Biol. Lett. 2013, 18, 368. |
| [20] | Chang, J.; Ren, H.; Zhao, M.; Chong, Y.; Zhao, W.; He, Y.; Qi, C. Eur. J. Med. Chem. 2017, 138, 669. |
| [21] | Li, S. Y.; Guo, C. Y.; Sun, X. Q.; Li, Y. Z.; Zhao, H. L.; Zhan, D. M.; Lan, M. B. Eur. J. Med. Chem. 2012, 49, 271. |
/
| 〈 |
|
〉 |