

Chinese Journal of Organic Chemistry >
One-Pot Three-Component Synthesis of 3-(1H-Benzo[d]imidazol-2-yl)chromen Derivatives
Received date: 2019-03-17
Online published: 2019-06-19
Supported by
the National Natural Science Foundation of China(21601061);the National Natural Science Foundation of China(51403073);the Department of Education of Jiangsu Province(16KJB150006);the Jiangsu Key Laboratory for Chemistry of Low-Dimensional Materials(JSKC15145)
Benzoimidazole and chromen derivatives exhibit a variety of important biological activities. Chromens incorporating benzoimidazole moiety have high Rho kinase inhibitory activity. However, the effective synthetic method for the preparation of these compounds is rare. The efficient synthesis of new substituted 3-(1H-benzo[d]imidazol-2-yl)-4H-chromens in 48%~89% yields via one-pot, three-component reaction of 2-(1H-benzo[d]imidazol-2-yl)acetonitrile with aromatic aldehydes and 5, 5-dimethylcyclohexane-1, 3-dione was studied. This reaction was carried out in EtOH in the presence of pyridine under reflux conditions. All reactions were completed within 1 to 3 h.
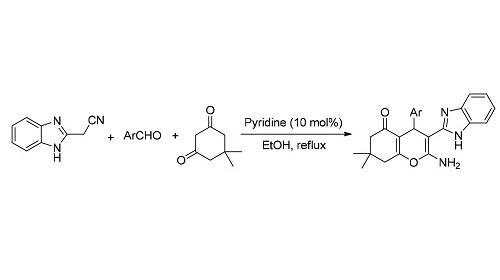
Key words: benzoimidazole; chromen; multicomponent reaction; heterocyclic skeleton
Xiang Wang , Ping Chen , Sanjun Zhi , Huayou Hu , Yuhe Kan , Zaichao Zhang . One-Pot Three-Component Synthesis of 3-(1H-Benzo[d]imidazol-2-yl)chromen Derivatives[J]. Chinese Journal of Organic Chemistry, 2019 , 39(11) : 3299 -3303 . DOI: 10.6023/cjoc201903032
| [1] | (a) Qiang, D. Z.; Shi, J. B.; Song, B. A.; Liu, X. H. RSC Adv. 2014, 4, 5607. |
| [1] | (b) Sashidhara, K. V.; Rosaiah, J. N.; Bhatia, G.; Saxena, J. K. Eur. J. Med. Chem. 2008, 43, 2592. |
| [1] | (c) Ding, X.; Xu, F. Chin. J. Org. Chem. 2018, 38, 3345 (in Chinese). |
| [1] | (丁晓友, 徐凡, 有机化学, 2018, 38, 3345. |
| [2] | (a) Akhtar, W.; Faraz Khan, M.; Verma, G.; Shaquiquzzaman, M.; Rizvib, M. A.; Hassan Mehdi, S.; Akhter, M.; Mumtaz Alam, M. Eur. J. Med. Chem. 2017, 126, 705. |
| [2] | (b) Bansal, Y.; Silakari, O. Bioorg. Med. Chem. Lett. 2012, 20, 6208. |
| [2] | (c) Yan, L.; Hou, Y.; Li, X.; Chen, H. Chin. J. Org. Chem. 2018, 38, 3332 (in Chinese). |
| [2] | (闫连海, 侯宇恒, 李小六, 陈华, 有机化学, 2018, 38, 3332.) |
| [2] | (d) Zhang, Z.; Zheng, X.; Guo, C. Chin. J. Org. Chem. 2016, 36, 1241 (in Chinese). |
| [2] | (张钊瑞, 郑晓霖, 郭长彬, 有机化学, 2016, 36, 1241.) |
| [2] | (e) Cheng, Z.; Zhang, Q. F.; Xu, X. L.; Li, X. N. Chin. J. Org. Chem. 2015, 35, 1189 (in Chinese). |
| [2] | (程正, 张群峰, 许孝良, 李小年, 有机化学, 2015, 35, 1189. |
| [3] | (a) Sessions, E. H.; Yin, Y.; Bannister, T. D.; Weiser, A.; Griffin, E.; Pocas, J.; Cameron, M. D.; Ruiz, C.; Lin, L.; Schürer, S. C.; Schr?ter, T.; LoGrasso, P.; Feng, Y. Bioorg. Med. Chem. Lett. 2008, 18, 6390. |
| [3] | (b) Sessions, E. H.; Smolinski, M.; Wang, B.; Frackowiak, B.; Chowdhury, S.; Yin, Y.; Chen, Y. T.; Ruiz, C.; Lin, L.; Pocas, J.; Schr?ter, T.; Cameron, M. D.; LoGrasso, P.; Feng, Y.; Bannister, T. D. Bioorg. Med. Chem. Lett. 2010, 20, 1939. |
| [4] | (a) Cioc, R. C.; Ruijter, E.; Orru, R. V. A. Green Chem. 2014, 16, 2958. |
| [4] | (b) Sunderhaus, J. D.; Dockendorff, C.; Martin, S. F. Org. Lett. 2007, 9, 4223. |
| [4] | (c) D?mling, A.; Wang, W.; Wang, K. Chem. Rev. 2012, 112, 3083. |
| [5] | Fadda A. A.; Youssif E. H. E. Synth. Commun. 2011, 41 677. |
| [6] | Wang X.; Chen P.; Zhi S.; Hu H.; Kan Y. Chin. J. Org. Chem. 2018, 38 3123. |
| [6] | 王 翔; 陈 平; 支 三军; 胡 华友; 阚 玉和 有机化学 2018, 38 3123. |
/
| 〈 |
|
〉 |