

Chinese Journal of Organic Chemistry >
Palladium-Catalyzed Thiazole-Directed mono-Selective C(sp2)-H Bond Iodination Reaction
Received date: 2019-04-25
Online published: 2019-06-19
Supported by
the National Science Foundation of China(21676088);the National Science Foundation of China(21476074)
A palladium-catalyzed ortho-C(sp2)-H bond iodination of 4-arylthiazoles has been developed. Through screening of directing groups and optimazation of reaction parameters, the most efficient reaction conditions for mono-ortho-position iodination were obtained, which were applied to synthesize a series of 4-(2-iodoaryl)thiazoles with broad scope of 4-aryl-thiazole substrates. Furthermore, the iodine group can be easily transformed into other organic functional groups, which improved the application value of this methodology. At last, plausible mechanism was proposed based on an intermolecular deuterium labeling kinetic experiment and radical inhibition experiments.
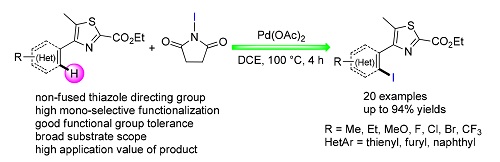
Key words: palladium-catalyzed; 4-arylthiazoles; mono-selectivity; C(sp2)-H bond; iodination
Lihao Xing , Lingyan Shao , Xiaopan Fu , Kezuan Deng , Jinyue Yang , Yafei Ji . Palladium-Catalyzed Thiazole-Directed mono-Selective C(sp2)-H Bond Iodination Reaction[J]. Chinese Journal of Organic Chemistry, 2019 , 39(11) : 3154 -3161 . DOI: 10.6023/cjoc201904062
| [1] | Gribble G. W. Acc. Chem. Res. 1998 31 141. |
| [2] | Lindley J. Tetrahedron 1984 40 1433. |
| [3] | Suzuki A. J. Organomet. Chem. 1999 576 147. |
| [4] | Crisp T. G. Chem. Soc. Rev. 1998 27 427. |
| [5] | Sambiagio C. Marsden S. P. Blacker A. J. McGowan P. C. Chem. Soc. Rev. 2014 43 3525. |
| [6] | Merkushev E. B. Synthesis 1988 923 |
| [7] | (a) Li, B.; Liu, B.; Shi, B. F. Chem. Commun. 2015, 51, 5093. |
| [7] | (b) Pal, P.; Singh, H.; Panda, A. B.; Ghosh, S. C. Asian J. Org. Chem. 2015, 4, 879. |
| [7] | (c) Zhan, B. B.; Liu, Y. H.; Hu, F.; Shi, B. F. Chem. Commun. 2016, 52, 4934. |
| [7] | (d) Aihara, Y.; Chatani, N. ACS Catal. 2016, 6, 4323. |
| [7] | (e) Khan, B.; Kant, R.; Koley, D. Adv. Synth. Catal. 2016, 358, 2352. |
| [7] | (f) Kommagalla, Y.; Yamazaki, K.; Yamaguchi, T.; Chatani, N. Chem. Commun. 2018, 54, 1359. |
| [7] | (g) Singh, H.; Sen, C.; Sahoo, T.; Ghosh, S. C. Eur. J. Org. Chem. 2018, 34, 4748. |
| [7] | (h) Du, Y.; Liu, Y. Y.; Wan, J. P. J. Org. Chem. 2018, 83, 3403. |
| [8] | Kalyani D. Dick A. R. Anani W. Q. Sanford M. S. Org. Lett. 2006 8 2523. |
| [9] | (a) Giri, R.; Chen, X.; Yu, J. Q. Angew. Chem., Int. Ed. 2005, 44, 2112. |
| [9] | (b) Li, J. J.; Mei, T. S.; Yu, J. Q. Angew. Chem., Int. Ed. 2008, 47, 6452. |
| [9] | (c) Mei, T. S.; Giri, R.; Maugel, N.; Yu, J. Q. Angew. Chem., Int. Ed. 2008, 47, 5215. |
| [9] | (d) Mei, T. S.; Wang, D. H.; Yu, J. Q. Org. Lett. 2010, 12, 3140. |
| [9] | (e) Nack, W. A.; Wang, X.; Wang, B.; He, G.; Chen, G. Beilstein J. Org. Chem. 2016, 12, 1243. |
| [9] | (f) Zhu, R. Y.; Liu, L. Y.; Yu, J. Q. J. Am. Chem. Soc. 2017, 139, 12394. |
| [9] | (g) Zhu, R. Y.; Saint-Denis, T. G.; Shao, Y.; He, J.; Sieber, J. D.; Senanayake, C. H.; Yu, J. Q. J. Am. Chem. Soc. 2017, 139, 5724. |
| [10] | (a) Dudnik, A. S.; Chernyak, N.; Huang, C.; Gevorgyan, V. Angew. Chem., Int. Ed. 2010, 49, 8729. |
| [10] | (b) Du, B.; Jiang, X.; Sun, P. J. Org. Chem. 2013, 78, 2786. |
| [10] | (c) Sadhu, P.; Alla, S. K.; Punniyamurthy, T. J. Org. Chem. 2013, 78, 6104. |
| [10] | (d) Pascanu, V.; Carson, F.; Solano, M. V.; Su, J.; Zou, X.; Johansson, M. J.; Martin-Matute, B. Chem. -Eur. J. 2016, 22, 3729. |
| [10] | (e) Testa, C.; Gigot, E.; Genc, S.; Decreau, R.; Roger, J.; Hierso, J. C. Angew. Chem., Int. Ed. 2016, 55, 5555. |
| [10] | (f) Yang, X.; Sun, Y.; Sun, T. Y.; Rao, Y. Chem. Commun. 2016, 52, 6423. |
| [10] | (g) Das, R.; Kapur, M. J. Org. Chem. 2017, 82, 1114. |
| [10] | (h) Dubost, E.; Babin, V.; Benoist, F.; Hebert, A.; Barbey, P.; Chollet, C.; Bouillon, J. P.; Manrique, A.; Pieters, G.; Fabis, F.; Cailly, T. Org. Lett. 2018, 20, 6302. |
| [10] | (i) Tang, R. J.; Milcent, T.; Crousse, B. J. Org. Chem. 2018, 83, 930. |
| [11] | (a) Chu, L.; Wang, X. C.; Moore, C. E.; Rheingold, A. L.; Yu, J. Q. J. Am. Chem. Soc. 2013, 135, 16344. |
| [11] | (b) Wang, X. C.; Hu, Y.; Bonacorsi, S.; Hong, Y.; Burrell, R.; Yu, J. Q. J. Am. Chem. Soc. 2013, 135, 10326. |
| [11] | (c) Chu, L.; Xiao, K. J.; Yu, J. Q. Science 2014, 346, 451 |
| [12] | (a) Lu, C.; Zhang, S. Y.; He, G.; Nack, W. A.; Chen, G. Tetrahedron 2014, 70, 4197. |
| [12] | (b) Chu, L.; Shang, M.; Tanaka, K.; Chen, Q.; Pissarnitski, N.; Streckfuss, E.; Yu, J. Q. ACS Cent. Sci. 2015, 1, 394. |
| [12] | (c) Sun, X.; Yao, X.; Zhang, C.; Rao, Y. Chem. Commun. 2015, 51, 10014. |
| [12] | (d) Fan, X. M.; Guo, Y.; Li, Y. D.; Yu, K. K.; Liu, H. W.; Liao, D. H.; Ji, Y. F. Asian J. Org. Chem. 2016, 5, 499. |
| [13] | (a) Das, R.; Kapur, M. Asian J. Org. Chem. 2018, 7, 1524. |
| [13] | (b) Liao, G.; Shi, B. F. Acta Chim. Sinica. 2015, 73, 1283 (in Chinese). |
| [13] | (廖港, 史炳烽, 化学学报, 2015, 73, 1283.) |
| [14] | Santra S. K. Banerjee A. Khatun N. Samanta A. Patel B. K. RSC Adv. 2015 5 11960. |
| [15] | (a) Al-Ghorbani, M.; Alghamdi, H. A.; Khanum, S. A. Eur. J. Biomed. Pharm. Sci. 2018, 5, 1. |
| [15] | (b) Attri, C.; Bhatia, P.; Kumar, P. J. Mod. Chem. Chem. Technol. 2018, 9, 19. |
| [15] | (c) Liaras, K.; Fesatidou, M.; Geronikaki, A. Molecules 2018, 23, 685/1. |
| [15] | (d) de Siqueira, L. R. P.; de Moraes Gomes, P. A. T.; de Lima Ferreira, L. P.; de Melo Rego, M. J. B.; Leite, A. C. L. Eur. J. Med. Chem. 2019, 170, 237. |
| [15] | (e) Scarim, C. B.; Jornada, D. H.; Machado, M. G. M.; Ferreira, C. M. R.; dos Santos, J. L.; Chung, M. C. Eur. J. Med. Chem. 2019, 162, 378. |
| [16] | Yu K. K. Guo Y. Hu Y. H. Xu Z. Liu H. W. Liao D. H. Ji Y. F. Asian J. Org. Chem. 2016 5 1219. |
| [17] | Bergstr m M. Suresh G. Naidu V. R. Unelius C. R. Eur. J. Org. Chem. 2017 2017 3234. |
| [18] | Qiao H. J. Yang F. Wang S. W. Leng Y. T. Wu Y. J. Tetrahedron 2015 71 9258. |
| [19] | Qiu F. C. Yang W. C. Chang Y. Z. Guan B. T. Asian J. Org. Chem. 2017 6 1361. |
/
| 〈 |
|
〉 |