

Chinese Journal of Organic Chemistry >
Bibenzyl Derivatives from Dendrobium nobile
Received date: 2019-03-20
Online published: 2019-07-03
Supported by
the National Natural Science Foundation of China(31560102);the Key Technologies R & D Program of Guizhou Province(黔科合重大专项字[2015]6010-2)
By silica gel, MCI column chromatographic and preparative high performance liquid chromatography (HPLC) technologies, three new bibenzyl derivatives were isolated from the tubes of Dendrobium nobile. One racemic compound was further purified by chiral (HPLC) to obtain a pair of enantiomers 1a and 1b, and the absolute configurations of the enantiomers were confirmed using electronic circular dichroism (ECD) calculations. By using spectroscopic techniques including NMR and HR-ESIMS, the structures of compounds 1~3 were identified as didendronbiline A (1), dendronbiline B (2), and dendronbiline C(3).
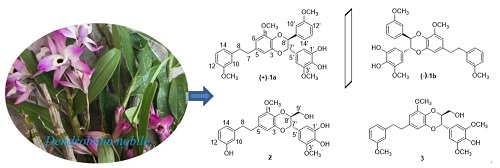
Key words: dendrobium nobile; orchidaceae; bisbibenzyl; dendronbiline
Maosheng Zhang , Lang Linghu , Jianyong Zhang , Xuqiang Nie , Xiaofei Li , Dale Guo , Shiji Xiao . Bibenzyl Derivatives from Dendrobium nobile[J]. Chinese Journal of Organic Chemistry, 2019 , 39(11) : 3289 -3293 . DOI: 10.6023/cjoc201903035
| [1] | Zhang H. Y. Zhang Z. Y. The Chinese Traditional Medicine Resource Records Beijing Science Press 1994 |
| [1] | 张 惠源 张 志英 中国中药资源志要 北京 科学出版社 1994 |
| [2] | Xu J. Han Q. B. Li S. L. Wang X. N. Zhao Z. Z. Chen H. B. Phytochem. Rev. 2013 12 341. |
| [3] | Zhang X. Q. Zhao T. M. Liu J. Zhao R. X. Zheng S. G. Ze C. Hu Y. D. Chin. Tradit. Herb. Drugs 2018 49 3174. |
| [3] | 张 雪琴 赵 庭梅 刘 静 赵 若茜 郑 世刚 淳 泽 胡 亚东 中草药 2018 49 3174. |
| [4] | Yang H. Sung S. H. Kim Y. C. J. Nat. Prod. 2007 70 1925. |
| [5] | Zhou X. M. Zheng C. J. Gan L. S. Chen G. Y. Zhang X. P. Song X. P. Li G. L. Sun C. G. J. Nat. Prod. 2016 79 1791. |
| [6] | Xiao S. J. Liu Z. Zhang M. S. Chen Y. Z. Nie X. Q. Zhang J. Y. He Y. Q. Shi J. S. Acta Pharm. Sin. 2016 51 1117. |
| [6] | 肖 世基 刘 珍 张 茂生 陈 永正 聂 绪强 张 建永 何 芋岐 石 京山 药学学报 2016 51 1117. |
| [7] | Xiao S. J. Qian Y. Zhang L. Tang Y. F. Zhu X. M. Zhou H. X. Zhao Z. N. Zhang M. S. Xu D. L. Chin. Tradit. Herb. Drugs 2016 47 2972. |
| [7] | 肖 世基 钱 怡 张 良 唐 艳芬 朱 雪梅 周 惠黠 赵 忠能 张 茂生 徐 德林 中草药 2016 47 2972. |
| [8] | Xiang T. Uno T. Ogino F. Ai C. Duo J. Sankawa U. Chem. Pharm. Bull. 2005 53 1204. |
| [9] | Lee D. Cuendet M. Vigo J. S. Graham J. G. Cabieses F. Fong H. H. Pezzuto J. M. Kinghorn A. D. Org. Lett. 2001 3 2169. |
| [10] | Liu Q. F. Chen W. L. Tang J. Zhao W. M. Helv. Chim. Acta 2007 90 1745. |
| [11] | Yang D. Cheng Z. Q. Yang L. Hou B. Yang J. Li X. N. Zi C. T. Dong F. W. Liu Z. H. Zhou J. Ding Z. T. Hu J. M. J. Nat. Prod. 2018 81 227. |
| [12] | Li Y. Wang C. L. Zhao H. J. Guo S. X. J. Asian Nat. Prod. Res. 2014 16 1035. |
| [13] | Li Y. Wang C. L. Wang Y. J. Wang F. F. Guo S. X. Yang J. S. Xiao P. G. Chem. Pharm. Bull. 2009 57 997. |
| [14] | Termentzi A. Zervou M. Kokkalou E. Food Chem. 2009 116 371. |
| [15] | Jiang S. Wang K. Lou H. Y. Yi P. Zhang N. Zhou M. Song Z. Q. Wang W. Wu M. K. Pan W. D. Phytochemistry 2019 162 216. |
| [16] | Xiao S. J. He D. H. Fang D. M. Chen F. Ding L. S. Zhou Y. Helv. Chim. Acta 2014 97 499. |
| [17] | Guo D. L. Li X. H. Feng D. Jin M. Y. Cao Y. M. Cao Z. X. Gu Y. C. Geng Z. Deng F. Deng Y. Molecules 2018 23 1709. |
| [18] | Liu H. S. Zhu G. L. Zhao S. G. Fu P. Zhu W. M. Chin. J. Org. Chem. 2019 39 507. |
| [18] | 刘 海珊 朱 国良 赵 水鸽 付 鹏 朱 伟明 有机化学 2019 39 507. |
/
| 〈 |
|
〉 |