

Chinese Journal of Organic Chemistry >
Design, Synthesis and Structure-Activity Relationships of Plant-Based 2-Aryl-3,4-dihydroisoquinolin-2-iums as Potential Antifungal Agents
Received date: 2019-05-10
Revised date: 2019-06-02
Online published: 2019-06-24
Supported by
Project supported by the National Natural Science Foundation of China (No. 21702173), the Fundamental Research Funds for the Central Universities (No. 2682016CX104) and the Miaozi Project of Scientific and Technological Innovation of Sichuan Province (No. 2018090).
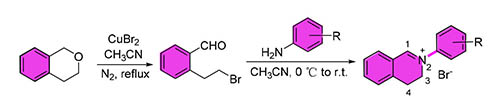
In order to discover more potent antifungal, a series of 2-aryl-3,4-dihydroisoquinolin-2-iums were reasonable designed and productive synthesized by introducing benzoic acid and phenol pharmacophores into the 2-position of isoquinoline. Their structures were identified by NMR and HRMS. The preliminary in vitro antifungal results showed that most of the title compounds exhibited moderate to significant inhibitory activities against various phytopathogenic fungi at 50 mg·mL-1, and were equal to controls (chlorothalonil, carbendazim). The concentration of 50% inhibition rate (EC50) of 2-(3-ethoxycarbonyl-phenyl)-3,4-dihydroisoquinolin-2-ium (4j) against R. solani was 3.8495 mg·mL-1, which was significantly superior to chlorothalonil (4.6328 mg·mL-1). All the EC50 values of 5 compounds (7.4583~15.4495 mg·mL-1) against R. cerealis were better than chlorothalonil (16.0137 mg·mL-1), and 2-(4-methoxycarbonylphenyl)-3,4-dihydroisoquinolin-2-ium (4f) is the best one. The present results provide valuable information for development of plant-based antifungal agents.
Chen Wei , Zuo Huailong , Li Yuxin , Liu Jiang , Zhou Xianli . Design, Synthesis and Structure-Activity Relationships of Plant-Based 2-Aryl-3,4-dihydroisoquinolin-2-iums as Potential Antifungal Agents[J]. Chinese Journal of Organic Chemistry, 2019 , 39(8) : 2317 -2322 . DOI: 10.6023/cjoc201905020
[1] Chen, W.; Li, Y. X.; Zhou, Y. Y.; Ma, Y.; Li, Z. M. Chin. Chem. Lett. 2019, DOI:10.1016/j.cclet.2019.04.072.
[2] Liang, R.; Li, X.; Yuan, W.; Jin, S.; Hou, S.; Wang, M.; Wang, H. J. Agric. Food Chem. 2018, 66, 9907.
[3] Hou, Z.; Zhu, L. F.; Yu, X. C.; Sun, M. Q.; Miao, F.; Zhou, L. J. Agric. Food Chem. 2016, 64, 2847.
[4] Eskola, M.; Altieri, A.; Galobart, J. World Mycotoxin J. 2018, 11, 277.
[5] Wu, Y. L.; Wang, D. L.; Guo, E. H.; Song, S.; Feng, J. T.; Zhang, X. Bioorg. Med. Chem. Lett. 2017, 27, 1284.
[6] Moss, S.; Ulber, L.; den Hoed, I. Crop Prot. 2019, 115, 13.
[7] Velioglu, Y. S.; Fikirdesici-Ergen, S.; Aksu, P.; Altindag, A. Tarim Bilim. Derg. 2018, 24, 245.
[8] Wei, Y. X.; Xu, X. Y.; Song, X. Curr. Org. Chem. 2017, 21, 1907.
[9] Mehta, S.; Sharma, K. Int. J. Pharma Sci. Res. 2016, 7, 4327.
[10] Ping, G.; Wang, Y.; Shen, L.; Wang, Y.; Hu, X.; Chen, J.; Hu, B.; Cui, L.; Meng, Q.; Li, C. Chem. Commun. 2017, 53, 7381.
[11] Ling, F.; Wu, Z. Q.; Jiang, C.; Liu, L.; Wang, G. X. J. Fish Dis. 2016, 39, 993.
[12] Lei, P.; Xu, Y.; Du, J.; Yang, X. L.; Yuan, H. Z.; Xu, G. F.; Ling, Y. Bioorg. Med. Chem. Lett. 2016, 26, 2544.
[13] Yang, R.; Gao, Z. F.; Zhao, J. Y.; Li, W. B.; Zhou, L.; Miao, F. J. Agric. Food Chem. 2015, 63, 1906.
[14] Thebault, M.; Mueller, U.; Kandelbauer, A.; Zikulnig-Rusch, E.; Lammer, H. Eur. J. Wood Wood Prod. 2017, 75, 853.
[15] Liu, C. L.; Yang, J. C. Handbook of Modern Pesticide, Chemical Industry Press, Beijing, 2017 (in Chinese). (刘长令,杨吉春,现代农药手册,化学工业出版社,北京, 2017.)
[16] Zhou, M. Y.; Kong, S. S.; Zhang, L. Q.; Zhao, M.; Duan, J. A.; Ou-yang, Z.; Wang, M. Tetrahedron Lett. 2013, 54, 3962.
[17] Ma, Y. N.; Yang, X. J.; Pan, L.; Hou, Z.; Geng, H. L.; Song, X. P.; Miao, F.; Zhou, L. Chem. Pharm. Bull. 2013, 61, 204.
/
| 〈 |
|
〉 |