

Chinese Journal of Organic Chemistry >
Eco-friendly C-3 Selenation of Imidazo[1,2-a]pyridines in Ionic Liquid
Received date: 2019-04-24
Revised date: 2019-06-12
Online published: 2019-07-09
Supported by
Project supported by the National Natural Science Foundation of China(21801007);The Program for Innovative Research Team of Science and Technology in the University of Henan Province(18IRTSTHN004);The Program for Innovative Research Team of Science and Technology in the University of Henan Province(18HASTIT006)
An relative eco-friendly protocol for the direct C-3 selenation of imidazo[1,2-a]pyridines with vorious organoselenides has been developed, gaving the desired products in moderate to excellent yields. Preliminary experimental results are consistent with a C-3 electrophilic functionalization mechanism. The features with relative green reaction conditions, broad substrate scope, and easy scale-up operation would make this strategy promising and important for the preparation of nitrogen and selenium containing molecules.
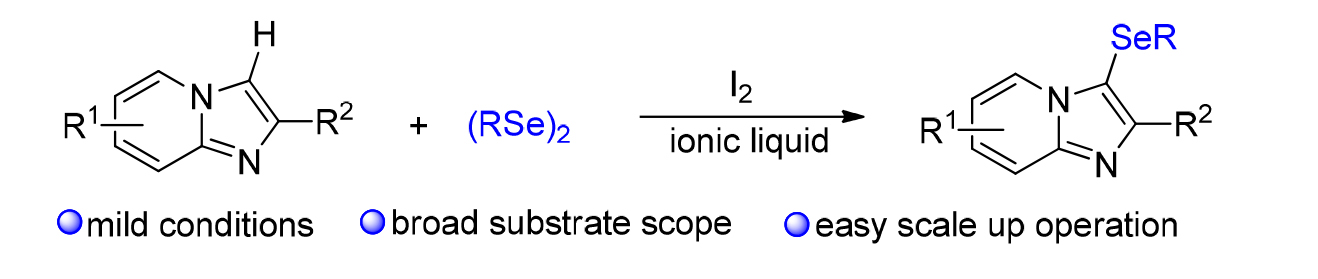
Xin Wang , Shiqiang Mu , Ting Sun , Kai Sun . Eco-friendly C-3 Selenation of Imidazo[1,2-a]pyridines in Ionic Liquid[J]. Chinese Journal of Organic Chemistry, 2019 , 39(10) : 2802 -2807 . DOI: 10.6023/cjoc201904057
| [1] | (a) Enguehard-Gueiffier, C.; Gueiffier, A. Mini-Rev. Med. Chem. 2007, 7, 888 |
| [1] | (b) Baviskar, A. T.; Amrutkar, S. M.; Trivedi, N.; Chaudhary, V.; Nayak, A.; Guchhait, S. K.; Banerjee, U. C.; Bharatam, P. V.; Kundu, C.N. ACS Med. Chem. Lett. 2015, 6, 481. |
| [2] | (a) Meng, T.; Wang, W.; Zhang, Z.; Ma, L.; Zhang, Y.; Miao, Z.; Shen, J. Bioorg. Med. Chem. 2014, 22, 848. |
| [2] | (b) Gallud, A.; Vaillantm, O.; Maillard, L. T.; Arama, D. P.; Dubois, J.; Maynadier, M.; Lisowski, V.; Garcia, M.; Martinez, J.; Masurier, N. Eur. J. Med. Chem. 2014, 75, 382. |
| [2] | (c) Zhao, Y.-X.; Ding, Y.-Y.; Lü, Y.-T.; Kang, C.-M. Chin. J. Org. Chem. 2019, 39, 1304.(in Chinese). |
| [2] | ( 赵鑫雨, 丁洋洋, 吕英涛, 康从民, 有机化学, 2019, 39, 1304.) |
| [3] | For selected papers, see: (a) Toure, B. B.; Lane, B. S.; Sames, D. Org. Lett. 2006, 8, 1979. |
| [3] | (b) Fu, H.; Chen, L.; Doucet, H. J. Org. Chem. 2012, 77, 4473. |
| [3] | (c) Choy, P. Y.; Luk, K. C.; Wu, Y.; So, C. M.; Wang, L.; Kwong, F.Y. J. Org. Chem. 2015, 80, 1457. |
| [4] | For selected papers, see: (a) Koubachi, J.; Kazzouli, S. E.; Berteina-Raboin, S.; Mouaddib, A.; Guillaumeta, G. Synthesis 2008,2537. |
| [4] | (b) Zhan, H.; Zhao, L.; Li, N.; Chen, L.; Liu, J.; Liao, J.; Cao, H. RSC Adv. 2014, 4, 32013. |
| [4] | (c) Ghosh, M.; Naskar, A.; Mitra, S.; Hajra, A. Eur. J. Org. Chem. 2015, 2015, 715. |
| [5] | Monir, K.; Bagdi, A. K.; Ghosh, M.; Hajra, A. J. Org. Chem. 2015, 80, 1332. |
| [6] | (a) Lei, S.; Chen, G.; Mai, Y.; Chen, L.; Cai, H.; Tan, J.; Cao, H. Adv. Synth. Catal. 2016, 358, 67. |
| [6] | (b) Gao, Y.; Lu, W.; Liu, P.; Sun, P. J. Org. Chem. 2016, 81, 2482. |
| [7] | Liu, P.; Gao, Y.; Gu, W.; Shen, Z.; Sun, P. J. Org. Chem. 2015, 80, 11559. |
| [8] | For selected papers see: (a) Ravi, C.; Mohan, D. C.; Adimurthy, S. . Org. Lett 2014, 16, 2978. |
| [8] | (b) Gao, Z.; Zhu, X.; Zhang, R. RSC. Adv. 2014, 4, 19891. |
| [8] | (c) Bagdi, A. K.; Mitra, S.; Ghosh, M.; Hajra, A. Org. Biomol. Chem. 2015, 13, 3314. |
| [8] | (d) Rafique, J.; Saba, Sumbal.; Rosário, A. R.; Braga, A.L. Chem. Eur. J. 2016, 22, 11854. |
| [8] | (e) Rafique, J.; Saba, S.; Franco, M. S.; Bettanin, L.; Schneider, A. R.; Silva, L. T.; Braga, A.L. Chem. Eur. J. 2018, 16, 880. |
| [8] | (f) Xie, L.-Y.; Peng, S.; Fan, T.-G.; Liu, Y.-F.; Sun, M.; Jiang, L.-L.; Wang, X.-X.; Cao, Z.; He, W.-M. Sci. China Chem. 2019, 62, 460. |
| [9] | For selected papers see: (a) Toure, B. B.; Lane, B. S.; Sames, D. Org. Lett. 2006, 8, 1979. |
| [9] | (b) Cao, H.; Zhan, H.; Lin, Y.; Lin, X.; Du, Z.; Jiang, H. Org. Lett. 2012, 14, 1688. |
| [9] | (c) Fu, H.; Chen, L.; Doucet, H. J. Org. Chem. 2012, 77, 4473. |
| [10] | For selected papers see : (a) Li, Z.; Hong, J.; Zhou, X. Tetrahedron 2011, 67, 3690. |
| [10] | (b) Ravi, C; Mohan, D. C.; Adimurthy, S. Org. Lett. 2014, 16, 2978. |
| [10] | (c) Liu, S.; Xi, H.; Zhang, J.; Wu, X.; Gao, Q.; Wu, A. Org. Biomol. Chem. 2015, 13, 8807. |
| [10] | (d) Huang, X.; Wang, S.; Li, B.; Wang, X.; Ge, Z.; Li, R. RSC Adv. 2015, 5, 22654. |
| [11] | For selected papers see: (a) Cao, H.; Lei, S.; Li, N.; Chen, L.; Liu, J.; Cai, H.; Qiu, S.; Tan, J. Chem. Commun. 2015, 51, 1823. |
| [11] | (b) Monir, K.; Bagdi, A. K.; Ghosh, M.; Hajra, A. J. Org. Chem. 2015, 80, 1332. |
| [11] | (c) Mitra, S.; Ghosh, M.; Mishra, S.; Hajra, A. J. Org. Chem. 2015, 80, 8275. |
| [11] | (d) Yang, D.; Yan, K.; Wei, W.; Li, G.; Lu, S.; Zhao, C.; Tian, L.; Wang, H. J. Org. Chem. 2015, 80, 11073. |
| [11] | (e) Sun, K.; Li, S.-J.; Chen, X.-L.; Liu, Y.; Huang, X.-Q.; Wei, D.-H.; Qu, L.-B.; Zhao, Y.-F.; Yu, B. Chem. Commun. 2019, 55, 2861. |
| [12] | For selected works see: (a) Mugesh, G.; du Mont, W. W.; Sies, H. Chem. Rev. 2001, 101, 2125. |
| [12] | (b) Mugesh, G.; Singh, H.B. Acc. Chem. Res. 2002, 35, 226. |
| [12] | (c) Nogueira, C. W.; Zeni, G.; Rocha, JB. T.. Chem. Rev. 2004, 104, 6255. |
| [12] | (d) Rhoden, C. R. B.; Zeni, G. Org. Biomol. Chem. 2011, 9, 1301. |
| [12] | (e) Liu, M.-X.; Li, Y.-M.; Yu, L.; Xu, Q.; Jiang, X.-F. Sci. China Chem. 2018, 6, 294. |
| [12] | (f) Lu, L.-H.; Zhou, S.-J.; He, W.-B.; Xia, W.; Chen, P.; Yu, X.; Xu, X.; He, W.-M. Org. Biomol. Chem. 2018, 16, 9064. |
| [12] | (g) Wu, C.; Xiao, H.-J.; Wang, S.-W.; Tang, M.-S.; Tang, Z.-L.; Xia, W.; Li, W.-F.; Cao, Z.; He, W.-M. ACS Sustainable Chem. Eng. 2019, 7, 2169. |
| [12] | (h) Feng, C.-L.; Zhu, J.; Tang, Q.-J.; Zhou, A.-H. Chin. J. Org. Chem. 2019, 39, 1187.(in Chinese). |
| [12] | ( 冯春来, 朱杰, 唐秋洁, 周爱华, 有机化学, 2019, 39, 1187.) |
| [13] | (a) Sun, K.; Wang, X.; Lv, Y.; Li, G.; Jiao, H.; Dai, C.; Li, Y.; Zhang, C.; Liu, L. Chem. Commun. 2016, 52, 8471. |
| [13] | (b) Sun, K.; ang, X.; Fu, F.; Zhang, C.; Chen, Y.; Liu, L. Green Chem. 2017, 19, 1490. |
| [13] | (c) Sun, K.; Lv, Y.; Shi, Z.; Fu, F.; Zhang, C.; Zhang, Z. Sci. China Chem. 2017, 60, 730. |
| [13] | (d) Sun, K.; Shi, Z.; Liu, Z.; Luan, B.; Zhu, J.; Xue, Y. Org. Lett. 2018, 20, 6687. |
| [13] | (e) Sun, K.; Wang, S.-N.; Feng, R.-R.; Zhang, Y.-X.; Wang, X.; Zhang, Z.-G.; Zhang, B. Org. Lett. 2019, 21, 2052. |
| [14] | Sun, K.; Wang, X.; Zhang, C.; Zhang, S.; Chen, Y.; Jiao, H.; Du, W. Chem. Asian J. 2017, 12, 713. |
| [15] | For selected recent works, see: (a) Rafique, J.; Saba, S.; Rosário, A. R.; Braga, A.L. Chem.-Eur. J. 2016, 22, 11854. |
| [15] | (b) Sun, P.-F.; Jiang, M.; Wei, W.; Min, Y.-Y.; Zhang, W.; Li, W.-H. Yang, D.-S.; Wang, H. J. Org. Chem. 2017, 82, 2906. |
| [15] | (c) Guo, T.; Wei, X.-N.; Wang, H.-Y.; Zhu, Y.-L.; Zhao, Y.-H.; Ma, Y.-C. Org. Biomol. Chem. 2017, 15, 9455. |
| [15] | (d) Guo, T.; Dong, Z.; Zhang, P.-K.; Xing, W.-Q.; Li, L.-P. Tetrahedron Lett. 2018, 59, 2554. |
| [15] | (e) Zhu, J.; Zhu, W.-H.; Xie, P.; Pittman, C. U.; Zhou, A.-H. Tetrahedron 2018, 74, 6569. |
| [15] | (f) Rafique, J.; Saba, S.; Franco, M. S.; Bettanin, L.; Schneider, A. R.; Silva, L. T.; Braga, A.L. Chem.-Eur. J. 2018, 24, 4173. |
| [15] | (g) Guo, T.; Wei, X.-N.; Liu, Y.; Zhang, P.-K.; Zhao, Y.-H. Org. Chem. Front. 2019, 6, 1414. |
| [16] | For selected example with ionic liquid as the solvent, see: (a) Xie, L.-Y.; Peng, S.; Lu, L.-H.; Hu, J.; Bao, W.-H.; Zeng, F.; Tang, Z.; Xu, X.; He, W.-M. ACS Sustainable Chem. Eng. 2018, 6, 7989. |
| [16] | (b) Wu, C.; Lu, L.-H.; Peng, A.-Z.; Jia, G.-K.; Peng, C.; Cao, Z.; Tang, Z.; He, W.-M.; Xu, X. Green Chem. 2018, 20, 3683. |
| [16] | (c) Yang, G.-P.; Wu, X.; Yu, B.; Hu, C. ACS Sustainable Chem. Eng. 2019, 7, 3727. |
/
| 〈 |
|
〉 |