

Chinese Journal of Organic Chemistry >
1,4-Diazabicyclo[2.2.2]octane (DABCO)-Catalyzed [4+2] Domino Reaction of Allenoates: Synthesis of Benzo[4,5]thieno-[3,2-b]pyran Derivatives
Received date: 2019-04-30
Revised date: 2019-06-25
Online published: 2019-07-09
Supported by
Project supported by the National Natural Science Foundation of China (No. 21403154), the Natural Science Foundation of Tianjin City (No. 17JCZDJC37700) and the Tianjin Municipal Education Commission (No. 20180KJ137).
A 1,4-diazabicyclo[2.2.2]octane (DABCO)-catalyzed[4+2] annulation reaction between 2-alkylidenebenzothio phene-3(2H)-ones and allenoate has been developed. The substrate scope includes both electron-withdrawing and electron-donating groups on the benzothiophene moiety. This method can be carried out under mild conditions and gives a wide range of highly functionalized benzothiophene-fused γ-pyran derivatives in good yields with moderate selectivity.
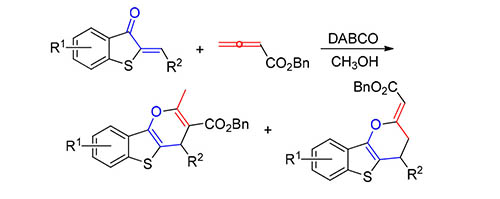
Key words: domino reaction; allenoate; benzothiophene derivatives
Jia Jiru , Yu Aimin , Liu Xuguang , Meng Xiangtai . 1,4-Diazabicyclo[2.2.2]octane (DABCO)-Catalyzed [4+2] Domino Reaction of Allenoates: Synthesis of Benzo[4,5]thieno-[3,2-b]pyran Derivatives[J]. Chinese Journal of Organic Chemistry, 2019 , 39(8) : 2175 -2182 . DOI: 10.6023/cjoc201904082
[1] (a) Scala, A.; Micale, N.; Piperno, A; Rescifina, A.; Schirmeister, T. Kesselring, J.; Grassi, G. RSC Adv. 2016, 6, 30628.
(b) Mishra, A.; Ma, C.-Q.; Bäuerle, P. Chem. Rev. 2009, 109, 1141.
(c) Rasmussen, S. C.; Evenson, S. J.; McCausland, C. B. Chem. Commun. 2015, 51, 4528.
(d) Gopalsamy, A.; Aplasca, A.; Ciszewski, G.; Park, K.; Ellingboe, J. W.; Orlowski, M.; Feld, B. Med. Chem. Lett. 2006, 16, 457.
[2] (a) Wright, J. B.; Johnson, H. G. J. Med. Chem. 1973, 16, 861.
(b) Gabbutt, C. D.; Heoworth, J. D.; Heron, B. M.; Thomas, J. L. Chem. Commun. 1999, 541.
[3] (a) Wang, Z.-G.; Yang, M.-F.; Yang, Y.-D. Org. Lett. 2018, 20, 3001.
(b) Tan, G.-Y.; You, Q.-L.; Lan, J.-B, You, J.-S. Angew. Chem., Int. Ed. 2018, 57, 6309.
(c) Qin, X.-R.; Li, X.-Y.; Huang, Q, Liu, H.; Wu, D.; Guo, Q.; Lan, J.-B.; Wang, R.-L.; You, J.-S. Angew. Chem. Int. Ed. 2015, 54, 7167.
[4] Pradhan, T. K.; De, A. Tetrahedron Lett. 2005, 46, 1493.
[5] (a) Caruana, L.; Forch, M.; Bernardi, L. Synlett. 2017, 28, 1530.
(b) Evans, C. S.; Davis, L. O. Molecules 2018, 23, 33.
(c) Chauhan, P.; Mahajan, S.; Enders, D. Acc. Chem. Res. 2017, 50, 2809.
(d) Volla, C. M.; Atodiresei, L.; Rueping, M. Chem. Rev. 2014, 114, 2390.
(e) Zheng, J.; He, M.; Xie, B.-H.; Yang, L.; Hu, Z.; Zhou, H.-B.; Dong, C. Org. Biomol. Chem. 2018, 16, 472.
[6] Ma, S.; Yu, A.; Zhang, L.; Meng, X. J. Org. Chem. 2018, 83, 5410.
[7] Ma, S.; Yu, A.; Meng, X. Org. Biomol. Chem. 2018, 16, 2885.
[8] (a) Jia, J.; Yu, A.; Ma, S.; Zhang, Y.; Li, K.; Meng, X. Org. Lett. 2017, 19, 6084.
(b) Zhang, Y.; Yu, A.; Jia, J.; Ma, S.; Li, K.; Wei, Y.; Meng, X. Chem. Commun. 2017, 53, 10672.
(c) Ding W.; Zhang, Y.; Yu, A.; Zhang, L.; Meng, X. J. Org. Chem. 2018, 83, 13821.
[9] Allenoate as one carbon synthon:(a) Meng, X.; Huang, Y.; Chen, R. Org. Lett. 2009, 11, 137.
(b) Xu, S.; Zhou, L.; Ma, R.; Song, H.; He, Z. Org. Lett. 2010, 12, 544.
[10] Selected examples of allenoate as two carbon synthon:(a) Gu, Y.; Li, F.; Hu, P.; Liao, D.; Tong, X. Org. Lett. 2015, 17, 1106.
(b) Yang, L.-J.; Li, S.; Wang, S.; Nie, J.; Ma, J.-A. J. Org. Chem. 2014, 79, 3547.
(c) Pei, C.-K.; Jiang, Y.; Wei, Y.; Shi, M. Angew. Chem., Int. Ed. 2012, 51, 11328.
(d) Liu, Y.; Du, Y.; Yu, A.; Qin, D.; Meng, X. Tetrahedron 2015, 71, 7706.
[11] Selected reviews for allenoates:(a) Li, Y.; Chen, X.; Chen, X.; Shen, X. Molecules 2018, 23, 3022.
(b) Chuang, S.-C.; Nallapati, B. S. Asian J. Org. Chem. 2018, 7, 1743.
(c) Wang, Q.-N.; Liu, Z. Q.; Lou, J, Yu, Z. Org. Lett. 2018, 20, 6007.
(d) Yu, Z.-Y.; Jin, Z.-C.; Duan, M, Bai, R.; Lu, Y.; Lan, Y. J. Org. Chem. 2018, 83, 9729.
(e) Vaishanv, N. K.; Gupta, A. K.; Kant, R.; Mohanan, K. J. Org. Chem. 2018, 83, 8759.
[12] Selected examples of allenoate as four carbon synthon:(a) Zhu, X.-F.; Lan, J.; Kwon, O. J. Am. Chem. Soc. 2003, 125, 4716.
(b) Villa, R. A.; Xu, Q.; Kwon, O. Org. Lett. 2012, 14, 4634.
(c) Wang, T.; Ye, S. Org. Lett. 2010, 12, 4168.
(d) Yuan, C.; Zhou, L.; Xia, M.; Sun, Z.; Wang, D.; Guo, H. Org. Lett. 2016, 18, 5644.
[13] CCDC-1913056(3a) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam. ac.uk/data_request/cif.
/
| 〈 |
|
〉 |