

Chinese Journal of Organic Chemistry >
Carbazolation Study of Active Arenes with Carbazole-Containing Hypervalent Iodine(III) Reagents
Received date: 2019-05-28
Revised date: 2019-07-12
Online published: 2019-07-17
Supported by
Project supported by the National Key Research and Development Program of China (No. 2016YFB0401400), the National Natural Science Foundation of China (Nos. 21302139, 21672120, 21871158) and the Fok Ying Tong Education Foundation of China (No. 151014).
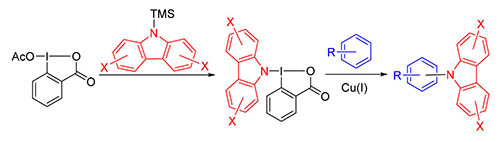
Carbazole and its derivatives are widely used in the field of medicine and photoelectric materials. A kind of stable cyclic hypervalent iodine reagents containing carbazole group was developed, which belong to benziodoxole compounds. In the presence of Cu(I) catalyst, these reagents reacted with aromatic substrates to give N-aryl carbazole derivatives. The reaction conditions are mild and suitable for a variety of electron-rich arenes. And a radical mechanism was proposed.
Key words: carbazole; hypervalent iodine reagent; N-arylation; copper-catalysis
Lan Tianlei , Zhang Yue , Liu Wei , Xi Chanjuan , Chen Chao . Carbazolation Study of Active Arenes with Carbazole-Containing Hypervalent Iodine(III) Reagents[J]. Chinese Journal of Organic Chemistry, 2019 , 39(8) : 2166 -2174 . DOI: 10.6023/cjoc201905050
[1] (a) Knölker, H.-J.; Reddy, K. R. Chem. Rev. 2002, 102, 4303.
(b) Schmidt, A. W.; Reddy, K. R.; Knölker, H.-J. Chem. Rev. 2012, 112, 3193.
[2] (a) Morin, J.-F.; Leclerc, M.; Adès D.; Siove, A. Macromol. Rapid Commun. 2005, 26, 761.
(b) Zhang, F.-F.; Zhou, C.-H.; Yan, J.-P. Chin. J. Org. Chem. 2010, 30, 783(in Chinese). (张飞飞,周成合,颜建平,有机化学, 2010, 30, 783.)
(c) Su, Y.-M.; Lin, H.-J.; Li, W.-M. Prog. Chem. 2015, 27, 1384(in Chinese). (苏玉苗,林海娟,李文木,化学进展, 2015, 27, 1384.)
(d) Sadiq, Z.; Hussain, E. A.; Naz, S. Mini-Rev. Org. Chem. 2017, 14, 469.
[3] (a) Ley, S. V.; Thomas A. W. Angew. Chem., Int. Ed. 2003, 42, 5400.
(b) Beletskaya, I. P.; Cheprakov, A. V. Coord. Chem. Rev. 2004, 248, 2337.
(c) Hartwig, J. F. Acc. Chem. Res. 2008, 41, 1534.
(d) Bariwalab, J.; Eycken E. V. Chem. Soc. Rev. 2013, 42, 9283.
(e) Heravi, M. M.; Kheilkordi, Z.; Zadsirjan, V.; Heydari M.; Malmir, J. Organomet. Chem. 2018, 861, 17.
[4] (a) Zhdankin, V. V.; Stang, P. J. Chem. Rev. 2002, 102, 2523.
(b) Zhdankin, V. V.; Stang, P. J. Chem. Rev. 2008, 108, 5299.
(c) Yoshimura, A.; Zhdankin, V. V. Chem. Rev. 2016, 116, 3328.
(d) Duan, Y.-N.; Jiang, S.; Han, Y.-C.; Sun, B.; Zhang, C. Chin. J. Org. Chem. 2016, 36, 1973(in Chinese). (段亚南,姜山,韩永超,孙博,张弛,有机化学, 2016, 36, 1973.)
[5] (a) Zhdankin, V. V. Curr. Org. Synth. 2005, 2, 121.
(b) Brand, J. P.; González, D. F.; Nicolai, S.; Waser, J. Chem. Commun. 2011, 47, 102.
(c) Waser, J. Synlett 2016, 27, 2761.
(d) Li, Y.-F.; Hari, D. P.; Vita, M. V.; Waser, J. Angew. Chem., Int. Ed. 2016, 55, 4436.
(e) Hari, D. P.; Caramenti P.; Waser, J. Acc. Chem. Res. 2018, 51, 3212.
[6] (a) Kieltsch, I.; Eisenberger, P.; Togni, A. Angew. Chem., Int. Ed. 2007, 46, 754.
(b) Parsons, A. T.; Buchwald, S. L. Angew. Chem., Int. Ed. 2011, 50, 9120.
(c) Mejía, E.; Togni, A. ACS Catal. 2012, 2, 521.
[7] (a) Brand, P. J.; Charpentier, J.; Waser J. Angew. Chem., Int. Ed. 2010, 48, 9346.
(b) Liu, X.; Wang, Z.; Cheng, X. J. Am. Chem. Soc. 2012, 134, 14330.
(c) Finkbeiner, P.; Kloeckner, U.; Nachtsheim, B. J. Angew. Chem., Int. Ed. 2015, 54, 4949.
[8] (a) Deng, Q.-H.; Bleith, T.; Wadepohl, H.; Dade, L. H. J. Am. Chem. Soc. 2013, 135, 5356.
(b) Sharma, A.; Hartwig, J. F. Nature 2015, 46, 600.
[9] Kiyokawa, K.; Kosaka, T.; Kojima, T.; Minakata, S. Angew. Chem., Int. Ed. 2015, 54, 13719.
[10] (a) Wang, H.; Zhang, D.; Sheng, H.; Bolm, C. J. Org. Chem. 2017, 82, 11854.(a) Wang, H.; Zhang, D.; Sheng, H.; Bolm, C. Chem.-Eur. J. 2018, 24, 14942.
[11] Lan, T.-L.; Qin, H.-J.; Chen, W.-T.; Liu, W.; Chen, C. Chin. Chem. Lett. 2019, DOI:10.1016/j.cclet.2019.07.031.
[12] (a) Cai, S.-J.; Chen, C.; Sun, Z.-L.; Xi, C.-J. Chem. Commun. 2013, 49, 4552.
(b) Gao, X.-Y.; Geng, Y.; Han, S.-J.; Liang, A.-P.; Li, J.-Y.; Zou, D.-P.; Wu, Y.-S.; Wu, Y.-J. Org. Lett. 2018, 20, 3732.
[13] Hsiao, S.-H.; Wang, H.-M. RSC Adv. 2016, 6, 43470
/
| 〈 |
|
〉 |