

Chinese Journal of Organic Chemistry >
Study on Synthetic Approach to 8-Hydroxyisocoumarin
Received date: 2019-06-03
Revised date: 2019-07-22
Online published: 2019-08-07
Supported by
Project supported by the National Natural Science Foundation of China (No. 31800678), the Shanxi Excellent Doctor Grant Award (No. SXYBKY201737) and the Science and Technology Innovation Project of Shanxi Agricultural University (No. 2017YJ38).
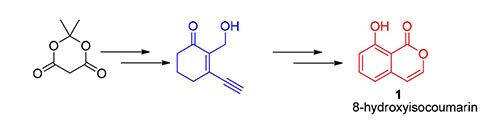
A new method for the synthesis of 8-hydroxyisocoumarin is reported. 8-Hydroxyisocoumarin was synthesized from Meldrum's acid by Friedel-Crafts acylation, carbonyl protection, ester reduction and deprotection. The key intermediate 3-ethynyl-2-(hydroxymethyl)cyclohex-2-en-1-one was synthesized by acidic cycloaddition, aromatization of cyclohexanone ring and 2,3-dicyano-5,6-dichlorobenzoquinone (DDQ) oxidation. The structures were characterized by 1H NMR, 13C NMR, IR, and HRMS.
Key words: isocoumarin; 8-hydroxyisocoumarin; pyran; aromatization; synthetic method
Guo Dongdong , Zhang Wuxia , Wang Yongqiang . Study on Synthetic Approach to 8-Hydroxyisocoumarin[J]. Chinese Journal of Organic Chemistry, 2019 , 39(9) : 2650 -2654 . DOI: 10.6023/cjoc201906001
[1] (a) Barry, R. D. Chem. Rev. 1964, 64, 229.
(b) Hill, R. A. Fortschritte der Chemie Organischer Naturstof-fe/Progress in the Chemistry of Organic Natural Products, Springer, Vienna, 1986, pp. 1~78.
[2] Fang, Z.-X.; Liu, J.-Y.; Qiao, Y.-H. Chin. J. Org. Chem. 2018, 38, 1985(in Chinese). (房智兴, 刘巨艳, 乔艳红, 有机化学, 2018, 38, 1985.)
[3] Dong, G.; Li, C.; Liu, H. Molecules 2019, 24, 937.
[4] Neog, K.; Dutta, D.; Das, B.; Gogoi, P. Org. Lett. 2017, 19, 730.
[5] Pal, S.; Chatare, V.; Pal, M. Curr. Org. Chem. 2011, 15, 782.
[6] Majetich, G.; Grove, J. L. Heterocycles 2012, 84, 983.
[7] Narasimhan, N. S.; Mali, R. S. Synthesis 1983, 957.
[8] Napolitano, E. Org. Prep. Proced. Int. 1997, 29, 631.
[9] Larock, R. C.; Doty, M. J.; Han, X. J. Org. Chem. 1999, 64, 8770.
[10] Larock, R. C.; Yum, E. K.; Doty, M. J.; Sham, K. K. C. J. Org. Chem. 1995, 60, 3270.
[11] Larock, R. C.; Varaprath, S.; Lau, H. H.; Fellows, C. A. J. Am. Chem. Soc. 1984, 106, 5274.
[12] Korte, D. E.; Hegedus, L. S.; Wirth, R. K. J. Org. Chem. 1977, 42, 1329.
[13] Cherry, K.; Parrain, J. L.; Thibonnet, J.; Duchêne, A.; Abarbri, M. J. Org. Chem. 2005, 70, 6669.
[14] Subramanian, V.; Batchu, V. R.; Barange, D.; Pal, M. J. Org. Chem. 2005, 70, 4778.
[15] Roy, S.; Roy, S.; Neuenswander, B.; Hill, D.; Larock, R. C. J. Comb. Chem. 2009, 11, 1128.
[16] Nayyar, N. K.; Hutchison, D. R.; Martinelli, M. J. J. Org. Chem. 1997, 62, 982.
[17] Organ, M. G.; Wang, J. J. Org. Chem. 2002, 67, 7847.
[18] Davidson, D.; Bernhard, S. A. J. Am. Chem. Soc. 1948, 70, 3426.
[19] Oikawa, Y.; Sugano, K.; Yonemitsu, O. J. Org. Chem. 1978, 43, 2087.
[20] Srikrishna, A.; Ramachary, D. B. Tetrahedron Lett. 2002, 43, 2765.
[21] Yamazaki, T.; Kobayashi, R.; Kitazume, T.; Kubota, T. J. Org. Chem. 2006, 71, 2499.
[22] Meyer, W. L.; Brannon, M. J.; Burgos, C. G. J. Org. Chem. 1985, 50, 438.
/
| 〈 |
|
〉 |