

Chinese Journal of Organic Chemistry >
Recent Advances in Asymmetric Functionalization of Olefins Induced by Chiral Hypervalent Iodine Reagents
Received date: 2020-06-09
Revised date: 2020-07-09
Online published: 2020-08-11
Supported by
the China Scholarship Council(201908620006); the Scientific Research Projects of Colleges and Universities in Gansu Province(2018B-091); the Teaching and Scientific Research Project of Lanzhou Petrochemical Polytechnic(JY2018-25)
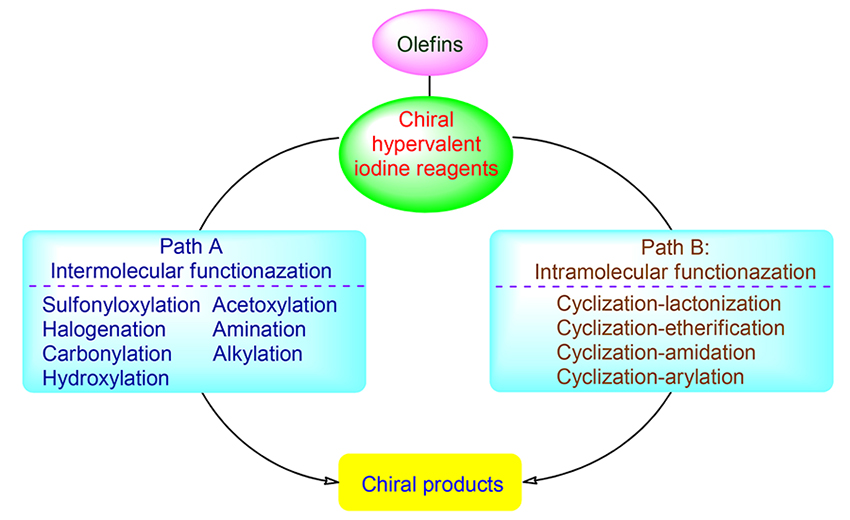
The functionalization of olefins induced by chiral hypervalent iodine reagents is a basic method for obtaining enantiomerically enriched chiral molecules and biologically active natural products. It is one of the new and promising fields in asymmetric synthesis. Throughout the past 20 years development in this area, on the one hand, stereoselective functionalizations of alkenes such as disulfonyloxylation, diacetoxylation, dihalogenation, diamination, hydroxylation-sulfonyloxylation and halogenation-alkylation and carbonylation have been successfully achieved with chiral hypervalent iodine reagents through intermolecular reactions, in which various functionalized products were enantioselectively obtained. On the other hand, many of hetero-cyclic compounds such as γ-butyrolactones, chromans, isochromans, oxazolidinones, piperidines, pyrrolidines and aziridines can be synthesized by intramolecular oxidative lactonization, etherification, amidation, arylation and other reactions, in which hypervalent iodine reagents can provide efficient methods for the synthesis of chiral heterocyclic compounds. In addition, various functionalization reactions of olefins mediated by chiral hypervalent iodine reagents can afford target compounds with excellent yield and enantioselectivity. Thus, this methodology has also been used in the total synthesis of natural products. The functionalization of olefins induced by chiral hypervalent iodine reagents and their applications in the total synthesis of natural products are reviewed.
Huaiyuan Zhang , Thomas Wirth . Recent Advances in Asymmetric Functionalization of Olefins Induced by Chiral Hypervalent Iodine Reagents[J]. Chinese Journal of Organic Chemistry, 2021 , 41(1) : 65 -70 . DOI: 10.6023/cjoc202006013
| [1] | Noyori R. Asymmetric Catalysis in Organic Synthesis, John Wiley & Sons, Chichester, 1994. |
| [1] | Ojima I. Catalytic Asymmetric Synthesis, Wiley-VCH, Weinheim, 2000. |
| [1] | Beller M.; Bolm C. Transition Metals for Organic Synthesis, Vol. 1, Wiley-VCH, Weinheim, 2004. |
| [1] | (d) Lin G.-Q.; Sun X.-W.; Chen Y.-Q.; Li Y.-M.; Chan A.S.C. Chiral Synthesis :A Symmetric Reaction and its Application, Science Press, Beijing, 2013. |
| [1] | ( 林国强, 孙兴文, 陈耀全, 李月明, 陈新滋, 手性合成: 不对称反应及其应用, 科学出版社, 北京, 2013. ). |
| [1] | (e) Carney J.R.; Dillon B.R.; Thomas S.P. Eur. J. Org. Chem. 2016, 3912. |
| [1] | (f) Romero N.A.; Nicewicz D.A. Chem. Rev. 2016, 116, 10075. |
| [1] | (g) Vekariya R.L. J. Mol. Liq. 2017, 227, 44. |
| [1] | (h) Srivastava V.; Singh P.P. RSC Adv. 2017, 7, 31377. |
| [1] | (i) Patel N.; Sood R.; Bharatam P.V. Chem. Rev. 2018, 118, 8770. |
| [1] | (j) Trowbridge A.; Walton S.M.; Gaun M.J. Chem. Rev. 2020, 120, 2613. |
| [1] | (k) Sheldon R.A.; Brady D.; Bode M.L. Chem. Sci. 2020, 11, 2587. |
| [2] | Jacobsen E.N.; Pfaltz A.; Yamamoto H. Comprehensive Asymmetric Catalysis, Springer, Berlin, 2000. |
| [2] | (b) Blaser H.-U.; Federsel H.-J. Asymmetric Catalysis on Industrial Scale: Challenges, Approaches and Solutions, Wiley-VCH, Weinheim, 2004. |
| [2] | (c) Krautwald S.; Carreira E.M. J. Am. Chem. Soc. 2017, 139, 5627. |
| [2] | (d) Bhattacharjee S.; Khan M.I.; Li X.; Zhu Q.-L.; Wu X.-T. Catalysts 2018, 8, 120. |
| [2] | (e) Lin Q.; Li L.; Luo S. Chem. -Eur. J. 2019, 25, 10033. |
| [2] | (f) Shaw S.; White J.D. Chem. Rev. 2019, 119, 9381. |
| [2] | (g) Wrzeszcz Z.; Siedlecka R. Molecules 2020, 25, 330. |
| [3] | Berkessel A.; Go?ger H. Asymmetric Organocatalysis :from Biomimetic Concepts to Applications in Asymmetric Synthesis, Wiley-VCH, Weinheim, 2005. |
| [3] | (b) Dalko P.I. Enantioselective Organocatalysis :Reactions and Experimental Procedures, Wiley-VCH, Weinheim, 2007. |
| [3] | (c) Erkkilä A.; Majander I.; Pihko P.M. Chem. Rev. 2007, 107, 5416. |
| [3] | (d) MacMillan D.W.C. Nature 2008, 455, 304. |
| [3] | (e) Barbas C.F. Angew. Chem., Int. Ed. 2008, 47, 42. |
| [3] | (f) Melchiorre P.; Marigo M.; Carlone A.; Bartoli G. Angew. Chem., Int. Ed. 2008, 47, 6138. |
| [3] | (g) Sun Y.-L.; Wei Y.; Shi M. ChemCatChem 2017, 9, 718. |
| [3] | (h) Nguyen T.N.; Chen P.-A.; Setthakarn K.; May J.A. Molecules 2018, 23, 2317. |
| [4] | (a) Adolfsson H. Angew. Chem., Int. Ed. 2005, 44, 3340. |
| [4] | (b) Ouellet S.G.; Walji A.M.; MacMillan D.W.C. Acc. Chem. Res. 2007, 40, 1327. |
| [4] | (c) Connon S.J. Org. Biomol. Chem. 2007, 5, 3407. |
| [4] | (d) Wang Z.; Jiang Z. Asian J. Chem. 2010, 22, 4141. |
| [4] | (e) de Vries, J.G.; Mršić, N. Catal. Sci. Technol. 2011, 1, 727. |
| [4] | (f) Rueping M.; Dufour J.; Schoepke F.R. Green Chem. 2011, 13, 1084. |
| [4] | (g) Phillips A.M.F.; Pombeiro A.J.L. Org. Biomol. Chem. 2017, 15, 2307. |
| [5] | (a) Wirth T. Hypervalent Iodine Chemistry in Topics in Current Chemistry, Vol.373, Springer, Switzerland, 2016. |
| [5] | (b) Zhdankin V.V.; Stang P.J. Chem. Rev. 2002, 102, 2523. |
| [5] | (c) Wirth T. Angew. Chem., Int. Ed. 2005, 44, 3656. |
| [5] | (d) Uyanik M.; Ishihara K. Chem. Commun. 2009, 2086. |
| [5] | (e) Merritt E.A.; Olofsson B. Angew. Chem., Int. Ed. 2009, 48, 9052. |
| [5] | (f) Zhdankin V.V. J. Org. Chem. 20 11, 76, 1185. |
| [5] | (g) González D.F.; Benfatti F.; Waser J. ChemCatChem 2012, 4, 955. |
| [5] | Singh F.V.; Wirth T. Synthesis 2013, 45, 2499. |
| [5] | (i) Zheng Z.S.; Zhang-Negrerie D.; Du Y.F.; Zhao K. Sci. China :Chem. 2014, 57, 189. |
| [5] | (j) Yoshimura A.; Zhdankin V.V. Chem. Rev. 2016, 116, 3328. |
| [5] | (k) Yoshimura A.; Yusubov M.S.; Zhdankin V.V. Org. Biomol. Chem. 2016, 14, 4771. |
| [5] | (l) Zhang H.; Tang R.; Wu J.; Hu Y. Chemistry 2018, 681. |
| [5] | ( 张怀远, 唐蓉萍, 伍家卫, 胡雨来, 化学通报, 2018, 681.). |
| [5] | (m) Reddy Kandimalla, S.; Prathima Parvathaneni, S.; Sabitha, G.; Subba Reddy, B.V. Eur. J. Org. Chem. 2019, 1687. |
| [5] | (n) Parra A. Chem. Rev. 2019, 119, 12033. |
| [5] | (o) Zhang H.; Tang R.; Shi X.; Xie L.; Wu J. Chin. J. Org. Chem. 2019, 39, 1837. |
| [5] | ( 张怀远, 唐蓉萍, 石星丽, 颉林, 伍家卫, 有机化学, 2019, 39, 1837.). |
| [5] | (p) Cai Q.; Ma H. Acta Chim. Sinica 2019, 77, 213. |
| [5] | ( 蔡倩, 马浩文, 化学学报, 2019, 77, 213.). |
| [5] | (q) Liu D.; He J.; Zhang C. Univ. Chem. 2019, 34, 1. |
| [5] | ( 刘丹, 贺家豪, 张弛, 大学化学, 2019, 34, 1.). |
| [6] | (a) Imamoto T.; Koto H. Chem. Lett. 1986, 15, 967. |
| [6] | (b) Ray III, D.G.; Koser, G.F. J. Am. Chem. Soc. 1990, 112, 5672. |
| [6] | (c) Xia M.; Chen Z.-C. Synth. Commun. 1997, 27, 1315. |
| [6] | (d) Tohma H.; Takizawa S.; Watanabe H.; Fukuoka Y.; Maegawa T.; Kita Y. J. Org. Chem. 1999, 64, 3519. |
| [6] | (e) Ladziata U.; Carlson J.; Zhdankin V.V. Tetrahedron Lett. 2006, 47, 6301. |
| [7] | (a) Ochiai M.; Kitagawa Y.; Takayama N.; Takaoka Y.; Shiro M. J. Am. Chem. Soc. 1999, 121, 9233. |
| [7] | (b) Companys S.; Peixoto P.A.; Bosset C.; Chassaing S.; Miqueu K.; Sotiropoulos J.-M.; Pouységu L.; Quideau S. Chem. -Eur. J. 2017, 23, 13309. |
| [8] | (a) Yu J.; Cui J.; Hou X.-S.; Liu S.-S.; Gao W.-C.; Jiang S.; Tian J.; Zhang C. Tetrahedron :Asymmetry 2011, 22, 2039. |
| [8] | (b) Mizar P.; Wirth T. Angew. Chem., Int. Ed. 2 014, 53, 5993. |
| [8] | (c) Suzuki S.; Kamo T.; Fukushi K.; Hiramatsu T.; Tokunaga E.; Dohi T.; Kita Y.; Shibata N. Chem. Sci. 2014, 5, 2754. |
| [8] | Brenet S.; Minozzi C.; Clarens B.; Amiri L.; Berthiol F. Synthesis 2015, 47, 3859. |
| [8] | (e) Basdevant B.; Legault C.Y. Org. Lett. 2015, 17, 4918. |
| [8] | (f) Cao Y.; Zhang X.; Lin G.; Zhang-Negrerie D.; Du Y. Org. Lett. 2016, 18, 5580. |
| [8] | (g) Pluta R.; Krach P.E.; Cavallo L.; Falivene L.; Rueping M. ACS Catal. 2018, 8, 2582. |
| [8] | (h) Wang Y.; Yuan H.; Lu H.; Zheng W.-H. Org. Lett. 2018, 20, 2555. |
| [9] | (a) Dohi T.; Maruyama A.; Takenaga N.; Senami K.; Minamitsuji Y.; Fujioka H.; Caemmerer S.B.; Kita Y. Angew. Chem., Int. Ed. 2008, 47, 3787. |
| [9] | (b) Dohi T.; Takenaga N.; Nakae T.; Toyoda Y.; Yamasaki Y.; Shiro M.; Fujioka H.; Maruyama A.; Kita Y. J. Am. Chem. Soc. 2013, 135, 4558. |
| [9] | (c) Uyanik M.; Yasui T.; Ishihara K. Angew. Chem., Int. Ed. 2013, 52, 9215. |
| [9] | (d) Bosset C.; Coffinier R.; Peixoto P.A.; El Assal M.; Miqueu K.; Sotiropoulos J.M.; Pouységu L.; Quideau S. Angew. Chem., Int. Ed. 2014, 53, 9860. |
| [9] | (e) Uyanik M.; Sasakura N.; Mizuno M.; Ishihara K. ACS Catal. 2017, 7, 872. |
| [9] | (f) Dohi T.; Sasa H.; Miyazaki K.; Fujitake M.; Takenaga N.; Kita Y. J. Org. Chem. 2017, 82, 11954. |
| [9] | (g) Hashimoto T.; Shimazaki Y.; Omatsu Y.; Maruoka K. Angew. Chem., Int. Ed. 2018, 57, 7200. |
| [9] | (h) Antien K.; Pouységu L.; Deffieux D.; Massip S.; Peixoto P.A.; Quideau S. Chem. -Eur. J. 2019, 25, 2852. |
| [10] | Malmedy F.; Wirth T. Chem. -Eur. J. 2016, 22, 16072. |
| [11] | (a) Castellanos, A; Fletcher, S.P.Chem. -Eur. J. 2011, 17, 5766. |
| [11] | (b) Verendel J.J.; Pàmies O.; Diéguez M.; Andersson P.G. Chem. Rev. 2014, 114, 2130. |
| [11] | (c) Coombs J.R.; Morken J.P. Angew. Chem., Int. Ed. 2016, 55, 2636. |
| [11] | (d) Margarita C.; Andersson P.G. J. Am. Chem. Soc. 2017, 139, 1346. |
| [11] | (e) Kraft S.; Ryan K.; Kargbo R.B. J. Am. Chem. Soc. 2017, 139, 11630. |
| [11] | (f) Fu X.; Zhao W. Chin. J. Org. Chem. 2019, 39, 625. |
| [11] | ( 付晓飞, 赵文献, 有机化学, 2019, 39, 625.). |
| [12] | Wirth T.; Hirt U.H. Tetrahedron : Asymmetry 1997, 8, 23. |
| [13] | Hirt U.H.; Spingler B.; Wirth T. J. Org. Chem. 1998, 63, 7674. |
| [14] | Hirt U.H.; Schuster M.F.H.; French A.N.; Wiest O.G.; Wirth T. Eur. J. Org. Chem. 20 01, 1569. |
| [15] | Koposov A.Y.; Boyarskikh V.V.; Zhdankin V.V. Org. Lett. 2004, 6, 3613. |
| [16] | (a) Ngatimin M.; Gartshore C.J.; Kindler J.P.; Naidu S.; Lupton D.W. Tetrahedron Lett. 2009, 50, 6008. |
| [16] | (b) Boppisetti J.K.; Birman V.B. Org. Lett. 2009, 11, 1221. |
| [17] | Fujita M.; Wakita M.; Sugimura T. Chem. Commun. 2 011, 47, 3983. |
| [18] | Shimogaki M.; Fujita M.; Sugimura T. Molecules 2015, 20, 17041. |
| [19] | Fujita M.; Miura K.; Sugimura T. Beilstein J. Org. Chem. 2018, 14, 659. |
| [20] | Ro?ben C.; Souto J.A.; González Y.; Lishchynskyi A.; Muñiz K. Angew. Chem., Int. Ed. 2011, 50, 9478. |
| [21] | Ro?ben C.; Souto J.A.; Escudero-Adán E.C.; Muñiz K. Org. Lett. 2013, 15, 1008. |
| [22] | Farid U.; Malmedy F.; Claveau R.; Albers L.; Wirth T. Angew. Chem., Int. Ed. 2013, 52, 7018. |
| [23] | Brown M.; Kumar R.; Rehbein J.; Wirth T. Chem. -Eur. J. 2016, 22, 4030. |
| [24] | Qurban J.; Elsherbini M.; Wirth T. J. Org. Chem. 2017, 82, 11872. |
| [25] | Hokamp T.; Wirth T. J. Org. Chem. 2019, 84, 8674. |
| [26] | Zhang D.-Y.; Zhang Y.; Wu H.; Gong L.-Z. Angew. Chem., Int. Ed. 2019, 58, 7450. |
| [27] | Ahmad A.; Silva Jr, L.F. J. Org. Chem. 2016, 81, 2174. |
| [28] | Jobin-Des Lauriers, A.; Legault, C.Y. Org. Lett. 2016, 18, 108. |
| [29] | Haubenreisser S.; Wo?ste T.H.; Martínez C.; Ishihara K.; Mun?iz K. Angew. Chem., Int. Ed. 2016, 55, 413. |
| [30] | Wo?ste T.H.; Mun?iz K. Synthesis 2016, 48, 816. |
| [31] | Molnár I.G.; Gilmour R. J. Am. Chem. Soc. 2016, 138, 5004. |
| [32] | Banik S.M.; Medley J.W.; Jacobsen E.N. J. Am. Chem. Soc. 2016, 138, 5000. |
| [33] | Haj M.K.; Banik S.M.; Jacobsen E.N. Org. Lett. 2019, 21, 4919. |
| [34] | Banik S.M.; Medley J.W.; Jacobsen E.N. Science 2016, 353, 51. |
| [35] | Mun?iz K.; Barreiro L.; Romero R.M.; Martínez C. J. Am. Chem. Soc. 2017, 139, 4354. |
| [36] | Sreenithya A.; Hadad C.M.; Sunoj R.B. Chem. Sci. 2019, 10, 7082. |
| [37] | Boye A.C.; Meyer D.; Ingison C.K.; French A.N.; Wirth T. Org. Lett. 2003, 5, 2157. |
| [38] | Fujita M.; Okuno S.; Lee H.J.; Sugimura T.; Okuyama T. Tetrahedron Lett. 2007, 48, 8691. |
| [39] | Fujita M.; Lee H.J.; Sugimura T.; Okuyama T. Chem. Commun. 2007, 1139. |
| [40] | Fujita M.; Yoshida Y.; Miyata K.; Wakisaka A.; Sugimura T. Angew. Chem., Int. Ed. 2010, 49, 7068. |
| [41] | (a) Fujita M.; Mori K.; Shimogaki M.; Sugimura T. Org. Lett. 2012, 14, 1294. |
| [41] | (b) Shimogaki M.; Fujita M.; Sugimura T. Eur. J. Org. Chem. 2013, 7128. |
| [42] | Deng Q.-H.; Wang J.-C.; Xu Z.-J.; Zhou C.-Y.; Che C.-M. Synthesis 2011, 2959. |
| [43] | Farid U.; Wirth T. Angew. Chem., Int. Ed. 2012, 51, 3462. |
| [44] | Kong W.; Feige P.; de Haro T.; Nevado C. Angew. Chem., Int. Ed. 2013, 52, 2469. |
| [45] | Mizar P.; Laverny A.; El-Sherbini M.; Farid U.; Brown M.; Malmedy F.; Wirth T. Chem. -Eur. J. 2014, 20, 9910. |
| [46] | Alhalib A.; Kamouka S.; Moran W.J. Org. Lett. 2015, 17, 1453. |
| [47] | Mizar P.; Niebuhr R.; Hutchings M.; Farooq U.; Wirth T. Chem. -Eur. J. 2016, 22, 1614. |
| [48] | Shimogaki M.; Fujita M.; Sugimura T. Angew. Chem., Int. Ed. 2016, 55, 15797. |
| [49] | Shimogaki M.; Fujita M.; Sugimura T. J. Org. Chem. 2017, 82, 11836. |
| [50] | Woerly E.M.; Banik S.M.; Jacobsen E.N. J. Am. Chem. Soc. 2016, 138, 13858. |
| [51] | Geary G.C.; Hope E.G.; Stuart A.M. Angew. Chem., Int. Ed. 2015, 54, 14911. |
| [52] | Mennie K.M.; Banik S.M.; Reichert E.C.; Jacobsen E.N. J. Am. Chem. Soc. 2018, 140, 4797. |
| [53] | Gelis C.; Dumoulin A.; Bekkaye M.; Neuville L.; Masson G. Org. Lett. 2017, 19, 278. |
| [54] | (a) Guimarães K.G.; Dias de Souza Filho, J.; Rennó dos Mares-Guia, T.; Castro Braga, F. Phytochemistry 2008, 69, 439. |
| [54] | (b) Endringer D.C.; Guimarães K.G.; Kondratyuk T.P.; Pezzuto J.M.; Braga F.C. J. Nat. Prod. 2008, 71, 1082. |
| [55] | Fujita M.; Mori K.; Shimogaki M.; Sugimura T. RSC Adv. 2013, 3, 17717. |
| [56] | Takesue T.; Fujita M.; Sugimura T.; Akutsu H. Org. Lett. 2014, 16, 4634. |
| [57] | (a) Garson M.J.; Staunton J.; Jones P.G. J. Chem. Soc., Perkin Trans. 1 1984, 1021. |
| [57] | (b) Krohn K.; Kock I.; Elsässer B.; Flörke U.; Schulz B.; Draeger S.; Pescitelli G.; Antus S.; Kurtán T. Eur. J. Org. Chem. 2007, 1123. |
/
| 〈 |
|
〉 |