

Chinese Journal of Organic Chemistry >
Synthesis, Crystal Structure and Antitumor Activity of Novel 5-Chloro-β-carboline Derivatives
Received date: 2020-06-15
Revised date: 2020-08-06
Online published: 2020-08-27
Supported by
the Scientific Research Innovation Project in Shihezi University(SHYL-YB201804); the Program for Changjiang Scholars and Innovative Research Team in University(IRT15R46); the Yangtze River Scholar Research Project of Shihezi University(CJXZ201601)
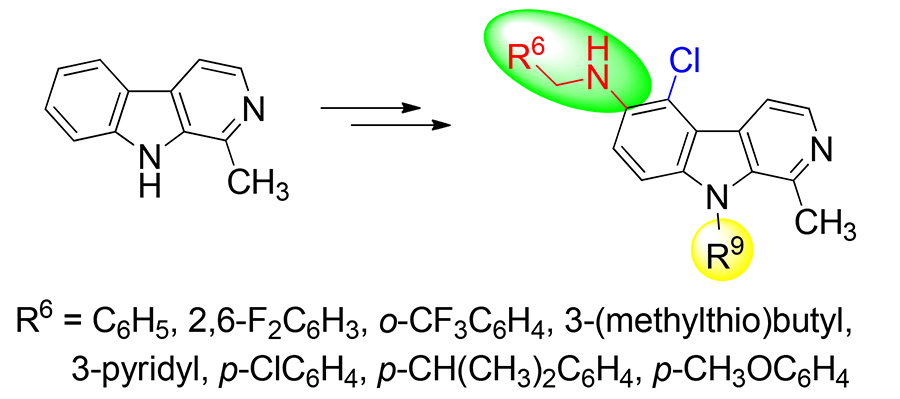
Sixteen novel 5-chloro- β-carboline derivatives were synthesized from harmane in four steps: N 9-alkylation, nitration, reduction, and Borch reduction. The structures of target compounds were confirmed by 1H NMR, 13C NMR, and HRMS. A single crystal of 5-chloro-1,9-dimethyl- N-(pyridin-3-ylmethyl)- β-carboline (5e) was cultured, and its single crystal structure was determined by X-ray diffraction study. The in vitro antiproliferative activities were evaluated in a panel of cancer cell lines (A549, BGC-823, CT-26, Bel-7402, and MCF-7) via methyl thiazolyl tetrazolium (MTT) assay. The results indicated that some compounds had good activities, and especially N-(2,6-difluorobenzyl)-1-methyl-5-chloro-9-(2,3,4,5,6-pentafluorobenzyl)- β- carboline-6-amine (5j) and N-(pyridin-3-ylmethyl)-1-methyl-5-chloro-9-(2,3,4,5,6-pentafluorobenzyl)- β-carboline-6-amine (5m) showed considerable antitumor activity with IC50 values lower than 10 μmol•L –1 against four cancer cell lines.
Yue Sun , Liang Guo , Wenxi Fan , Wei Chen , Jie Zhang , Bin Dai . Synthesis, Crystal Structure and Antitumor Activity of Novel 5-Chloro-β-carboline Derivatives[J]. Chinese Journal of Organic Chemistry, 2021 , 41(1) : 400 -406 . DOI: 10.6023/cjoc202006026
| [1] | Bray F.; Ferlay J.; Soerjomataram I.; Siegel R.L.; Torre L.A.; Jemal A. CA-Cancer J. Clin. 2018, 68, 394. |
| [2] | Ling Y.; Feng J.; Luo L.; Guo J.; Peng Y.-F.; Wang T.-T.; Ge X.; Xu Q.-B.; Wang X.-Y.; Dai H.; Zhang Y.-N. ChemMedChem 2017, 12, 646. |
| [3] | Akabli T.; Lamchouri F.; Senhaji S.; Oufik H. Struct. Chem. 2019, 30, 4. |
| [4] | Sarragiotto M.; Brand G.; Gomes C.; Costa W.; Foglio M.; Ruiz A. Synthesis 2019, 51, 573. |
| [5] | Priya Jeyapal, G.; Krishnasamy, R.; Suzuki, C.K.; Venkatesh, S.; Chandrasekar, M. Bioorg. Chem. 2019, 88, 102913. |
| [6] | Ashok P.; Chander S.; Balzarini J.; Pannecouque C.; Murugesan S. Bioorg. Med. Chem. Lett. 2015, 25, 1232. |
| [7] | Gorki V.; Singh R.; Walter N.S.; Bagai U.; Salunke D.B. ACS Omega 2018, 3, 13200. |
| [8] | Chatwichien J.; Basu S.; Murphy M.E.; Hamann M.T.; Winkler J.D. Tetrahedron Lett. 2015, 56, 3515. |
| [9] | Laine A.; Lood C.; Koskinen A. Molecules 2014, 19, 1544. |
| [10] | Khan H.; Patel S.; Kamal M.A. Curr. Drug Metab. 2017, 18, 853. |
| [11] | Saini K.; Singh J.; Shah R.; Kaur J.; Singh D.; Singh N.; Singh J.A.; Chopra D.S.; Singh R.S. Med. Chem. Res. 2020, 29, 1400. |
| [12] | Foley C.A.; Al-Issa Y.A.; Hiller K.P.; Mulcahy S.P. ACS Omega 2019, 4, 9807. |
| [13] | Lu X.; Liu Y.-C.; Orvig C.; Liang H.; Chen Z.-F. Eur. J. Med. Chem. 2019, 181, 111567. |
| [14] | Audia J.E.; Droste J.J.; Nissen J.S.; Murdoch G.L.; Evrard D.A. J. Org. Chem. 1996, 61, 7937. |
| [15] | Movassaghi M.; Hill M.D. Org. Lett. 2008, 10, 3485. |
| [16] | Shen T.; Zhu B.-C.; Lin F.-G.-R.; Pan J.; Wei J.-L.; Luo X.; Liu J.-Z.; Jiao N. Chin. J. Chem. 2018, 36, 815. |
| [17] | Wang Z.; Yu Z.-Z.; Yao Y.; Zhang Y.-K.; Xiao X.-F.; Wang B. Chin. Chem. Lett. 2019, 30, 1541. |
| [18] | Wang R.-X. M.S. Thesis, Northwest A&F University, Shanxi, 2018. (in Chinese) |
| [18] | ( 王瑞雪, 硕士论文, 西北农林科技大学, 陕西,20 18. ). |
| [19] | Lunagariya N.A.; Gohil V.M.; Kushwah V.; Neelagiri S.; Jain S.; Singh S.; Bhutani K.K. Bioorg. Med. Chem. Lett. 2016, 26, 789. |
| [20] | Chen, Z.-Y,; Cao, R.-H.; Shi, B.; Guo, L.; Sun, J.; Ma, Q.; Fan, W.-X.; Song, H.-C. Eur. J. Med. Chem. 2011, 46, 5127. |
| [21] | Zhang G.-X.; Cao R.-H.; Guo L.; Ma Q.; Fan W.-X.; Chen X.-M.; Li J.-R.; Shao G.; Qiu L.-Q.; Ren Z.-H. Eur. J. Med. Chem. 2013, 65, 21. |
| [22] | Guo L.; Fan W.-X.; Chen X.-M.; Ma Q.; Cao R.-H. Chin. J. Org. Chem. 2013, 33, 332. (in Chinese) |
| [22] | ( 郭亮, 范文玺, 陈雪梅, 马芹, 曹日晖, 有机化学, 2013, 33, 332.). |
| [23] | Guo L.; Fan W.-X.; Gan Z.-Y.; Chen W.; Ma Q.; Cao R.-H. J. Chin. Pharm. Sci. 2015, 24, 801. |
| [24] | Guo L.; Xie J.-W.; Fan W.-X.; Chen W.; Dai B.; Ma Q. Chin. J. Org. Chem. 2017, 37, 1741. (in Chinese) |
| [24] | ( 郭亮, 谢建伟, 范文玺, 陈伟, 代斌, 马芹, 有机化学, 2017, 37, 1741.). |
| [25] | Guo L.; Cao R.-H.; Fan W.-X.; Gan Z.-Y.; Ma Q. Chem. J. Chin. Univ. 2016, 37, 1093. (in Chinese) |
| [25] | ( 郭亮, 曹日晖, 范文玺, 甘紫云, 马芹, 高学校化学学报, 2016, 37, 1093.). |
| [26] | Chen W.; Zhang G.-X.; Guo L.; Fan W.-X.; Ma Q.; Zhang X.-D.; Du R.-L.; Cao R.-H. Eur. J. Med. Chem. 2016, 124, 249. |
| [27] | Guo L.; Chen W.; Cao R.-H.; Fan W.-X.; Ma Q.; Zhang J.; Dai B. Eur. J. Med. Chem. 2018, 147, 253. |
| [28] | Guo L.; Ma Q.; Chen W.; Fan W.-X.; Zhang J.; Dai B. J. Enzyme Inhib. Med. Chem. 2019, 34, 375. |
| [29] | Guo L.; Chen X.-F.; Chen W.; Ma Q.; Fan W.-X.; Zhang J.; Dai B. Bioorg Chem. 2020, 96, 103612. |
| [30] | Ma C.-M.; Cao R.-H.; Shi B.-X.; Zhou X.-T.; Ma Q.; Sun J.; Guo L.; Yi W.; Chen Z.-Y.; Song H.-C. Eur. J. Med. Chem. 2010, 45, 5513. |
| [31] | Wang S.-Q.; Huang W.-Y.; Zhang X.-R.; Zhang X.-T.; Pan C.-X. Chin. J. Org. Chem. 2020, 40, 959. (in Chinese) |
| [31] | ( 王淑琴, 黄婉云, 张小蓉, 张晓婷, 潘成学, 有机化学, 2020, 40, 959.). |
| [32] | Wu B.-W.; Cui X.-X.; Zhu T.; Wang S.-H.; Lu C.-F.; Wang J.-J.; Dang H.-X.; Zhang S.-Y.; Ding L.-N.; Jin C.-Y. Chin. J. Org. Chem. 2020, 40, 978. (in Chinese) |
| [32] | ( 吴博文, 崔鑫鑫, 朱挺, 王胜辉, 陆超凡, 王金杰, 党贺祥, 张赛扬, 丁丽娜, 金成允, 有机化学, 2020, 40, 978). |
/
| 〈 |
|
〉 |